|
[1]
|
CONLEY D J, PAERL H W, HOWARTH R W, et al.Controlling eutrophication: Nitrogen and phosphorus[J].Science,2009,323(5917):1014–1015 10.1126/science.1167755
|
|
[2]
|
PAN B, WU J, PAN B, et al.Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents[J].Water Research,2009,43(17):4421–4429 10.1016/j.watres.2009.06.055
|
|
[3]
|
LU S G, BAI S Q, ZHU L, et al.Removal mechanism of phosphate from aqueous solution by fly ash[J].Journal of Hazardous Materials,2009,161(1):95-101 10.1016/j.jhazmat.2008.02.123
|
|
[4]
|
YE J, CONG X, ZHANG P, et al.Preparation of a new granular acid-activated neutralized red mud and evaluation of its performance for phosphate adsorption[J].ACS Sustainable Chemistry & Engineering,2015,3(12):3324–3331 10.1021/acssuschemeng.5b00932
|
|
[5]
|
ZENG L, LI X, LIU J.Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings[J].Water Research,2004,38(5):1318–1326 10.1016/j.watres.2003.12.009
|
|
[6]
|
ZENG H, FISHER B, GIAMMAR D E.Individual and competitive adsorption of arsenate and phosphate to a high-surface-area iron oxide-based sorbent[J].Environmental Science & Technology,2008,42(1):147–152 10.1021/es071553d
|
|
[7]
|
WU K, LIU T, MA C, et al.The role of Mn oxide doping in phosphate removal by Al-based bimetal oxides: Adsorption behaviors and mechanisms[J].Environmental Science & Pollution Research International,2014,21(1):620–630 10.1007/s11356-013-1937-x
|
|
[8]
|
李海宁, 陈静, 李秋梅,等. 铁锰复合氧化物包覆海砂的吸附除磷研究[J]. 环境科学学报, 2016,36(3):880–886 10.13671/j.hjkxxb.2015.0458
|
|
[9]
|
SAHARAN P, CHAUDHARY G R, MEHTA S K, et al.Removal of water contaminants by iron oxide nanomaterials[J].Journal of Nanoscience & Nanotechnology,2014,14(1):627–643 10.1166/jnn.2014.9053
|
|
[10]
|
LIU C, BAI R.Recent advances in chitosan and its derivatives as adsorbents for removal of pollutants from water and wastewater[J].Current Opinion in Chemical Engineering,2014,4:62–70 10.1016/j.coche.2014.01.004
|
|
[11]
|
HE J, BARDELLI F, GEHIN A, et al.Novel chitosan goethite bionanocomposite beads for arsenic remediation[J].Water Research,2016,101:1–9 10.1016/j.watres.2016.05.032
|
|
[12]
|
YAMAIN J S, LOUNSBURY A W, ZIMMERMAN J B.Adsorption of selenite and selenate by nanocrystalline aluminum oxide, neat and impregnated in chitosan beads[J].Water Research,2014,50(3):373–381 10.1016/j.watres.2013.10.054
|
|
[13]
|
JIANG H, CHEN P, LUO S, et al.Synthesis of novel biocompatible composite Fe3O4/ZrO2/chitosan and its application for dye removal[J].Journal of Inorganic & Organometallic Polymers & Materials,2013,23(2):393–400 10.1007/s10904-012-9792-7
|
|
[14]
|
闵伶俐, 郑煜铭, 钟鹭斌,等. 铁氧化物/壳聚糖复合纳米纤维的制备及吸附五价砷研究[J]. 环境科学学报,2014,34(12):2979–2984 10.13671/j.hjkxxb.2014.0688
|
|
[15]
|
SOWMYA A, MEENAKSHI S.Zr(IV) loaded cross-linked chitosan beads with enhanced surface area for the removal of nitrate and phosphate[J].International Journal of Biological Macromolecules,2014,69(8):336–343 10.1016/j.ijbiomac.2014.05.043
|
|
[16]
|
LAGERGREEN S.Zur theorie der sogenannten adsorption gel?ster stoffe[J].Zeitschrift Für Chemie Und Industrie Der Kolloide,1907,2(1):15
|
|
[17]
|
HO Y S, MCKAY G, WASE D A J, et al.Study of the sorption of divalent metal ions on to peat[J].Adsorption Science & Technology,2000,18(7):639–650 10.1260/0263617001493693
|
|
[18]
|
CHIEN S H, CLAYTON W R.Application of Elovich equation to the kinetics of phosphate release and sorption in soils[J].Soil Science Society of America Journal,1980, 44(2):265–268 10.2136/sssaj1980.03615995004400020013x
|
|
[19]
|
WEBER W J, MORRIS J C.Kinetics of adsorption on carbon from solution[J].Asce Sanitary Engineering Division Journal,1963,1(2):1–2
|
|
[20]
|
王永亮. 壳聚糖水凝胶诱导合成纳米Fe3O4及同心层状磁性水凝胶构建[D]. 哈尔滨:哈尔滨工业大学, 2011
|
|
[21]
|
MOHAPATRA M, HARIPRASAD D, MOHAPATRA L, et al.Mg-doped nano ferrihydrite:A new adsorbent for fluoride removal from aqueous solutions[J].Applied Surface Science,2012,258(10):4228–4236 10.1016/j.apsusc.2011.12.047
|
|
[22]
|
付军, 范芳, 李海宁,等. 铁锰复合氧化物/壳聚糖珠:一种环境友好型除磷吸附剂[J]. 环境科学, 2016,137(12):4882–4890 10.13227/j.hjkx.201608168
|
|
[23]
|
丁伟, 巴图其木格, 张玲玲,等. 自燃煤矸石吸附磷的动力学和热力学[J]. 环境工程学报, 2017,11(7):4059–4066 10.12030/j.cjee.201604165
|
|
[24]
|
OLIVEIRA M, ARAUJO A, AZEVEDO G, et al.Kinetic and equilibrium studies of phosphorous adsorption: Effect of physical and chemical properties of adsorption agent[J].Ecological Engineering,2015,82:527–530 10.1016/j.ecoleng.2015.05.020
|
|
[25]
|
程翔.类水滑石吸附和蓝铁石沉淀回收污水中磷的研究[D].哈尔滨:哈尔滨工业大学,2010
|
|
[26]
|
CHEUNG W H, SZETO Y S, MCKAY G.Intraparticle diffusion processes during acid dye adsorption onto chitosan[J].Bioresource Technology,2007,98(15):2897–2904 10.1016/j.biortech.2006.09.045
|
|
[27]
|
TIAN S, JIANG P, PING N, et al.Enhanced adsorption removal of phosphate from water by mixed lanthanum/aluminum pillared montmorillonite[J].Chemical Engineering Journal,2009,151(1/2/3):141–148 10.1016/j.cej.2009.02.006
|
|
[28]
|
BISWAS B K, INOUE K, GHIMIRE K N, et al.The adsorption of phosphate from an aquatic environment using metal-loaded orange waste[J].Journal of Colloid and Interface Science,2007,312(2):214–223 10.1016/j.jcis.2007.03.072
|
|
[29]
|
王慧. 铁氧化物及其胡敏酸复合体对磷酸盐的吸附研究[D]. 武汉:华中农业大学, 2015 10.7666/d.Y2803159
|
|
[30]
|
周宏光. 载纳米水合氧化铁复合半焦的研制及其除磷性能研究[D]. 重庆:西南大学, 2014
|
|
[31]
|
黄微雅. 功能化改性无机除磷吸附剂的制备及吸附性能研究[D]. 广州:暨南大学, 2013
|


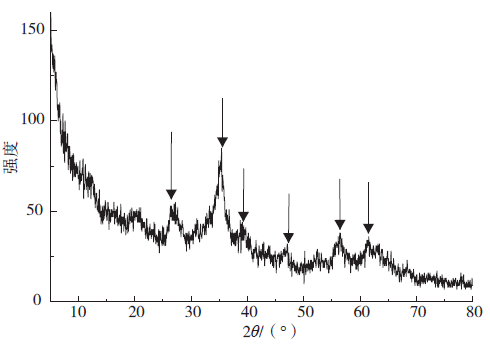



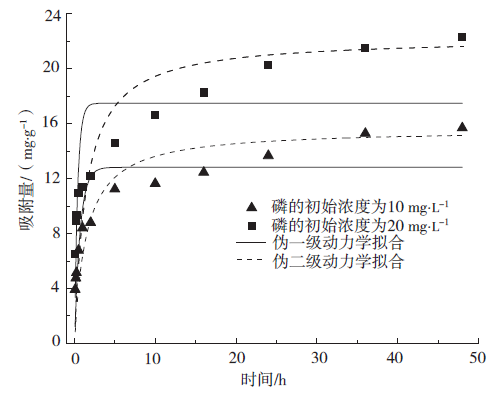










 下载:
下载:
