-
20世纪90年代,海洋环境中发现大量塑料碎片,这些碎片能够缠绕海洋生物并被当作食物摄入[1],同时还可充当载体将其他环境污染物转运至生物体内[2]. 微塑料(microplastics,MPs)于2004年首先被Thompson等提出[3],通常指粒径小于5 mm的塑料碎片. 当前对微塑料的研究主要包括环境微塑料赋存特征,微塑料污染控制及降解技术,微塑料毒理与生态风险评估等内容. 早期关于微塑料的研究范围主要集中在水环境领域[4 − 6],近年来发现陆地环境中的微塑料暴露更为严重,可能是海洋环境的4—23倍[7 − 8],大气[9]微塑料的研究也逐渐展开,暴露研究逐渐由海洋生物转向陆地植物、动物乃至人类,微塑料生物暴露引发的生态与人类健康问题,特别是在组织器官损伤、氧化应激、炎症、神经毒性等方面成为研究热点. 基于“肠-脑轴”在生理过程中发挥的重要作用以及肠道菌群在其中扮演的重要角色,近年来微塑料暴露涉及“肠-脑轴”的相关研究也逐渐得到关注,然而目前关于这方面开展的系统研究较少. 本文综述了近年来国内外关于微塑料暴露对肠道菌群、神经毒性及有关机制的研究,为进一步深入探讨微塑料暴露对“肠道菌群-肠-脑轴”的潜在毒性影响与机制、寻找可能存在的生物标志物、保护生物预防或减轻微塑料暴露危害提供研究思路.
-
微塑料在环境中几乎无处不在[10 − 12],其主要来源包括被直接制造成微小尺寸作为原材料添加到化妆品、洗面奶等产品中的初级微塑料,及宏观塑料在环境中破碎分解产生的次级微塑料[13]. 微塑料在环境中进一步分解可产生纳米级的塑料碎片, Gigault等[14]针对前人研究中关于纳米塑料的争论,提出纳米塑料(nanoplastics,NPs)是由工业塑料物体降解、或在塑料产品使用过程中产生的尺寸小于
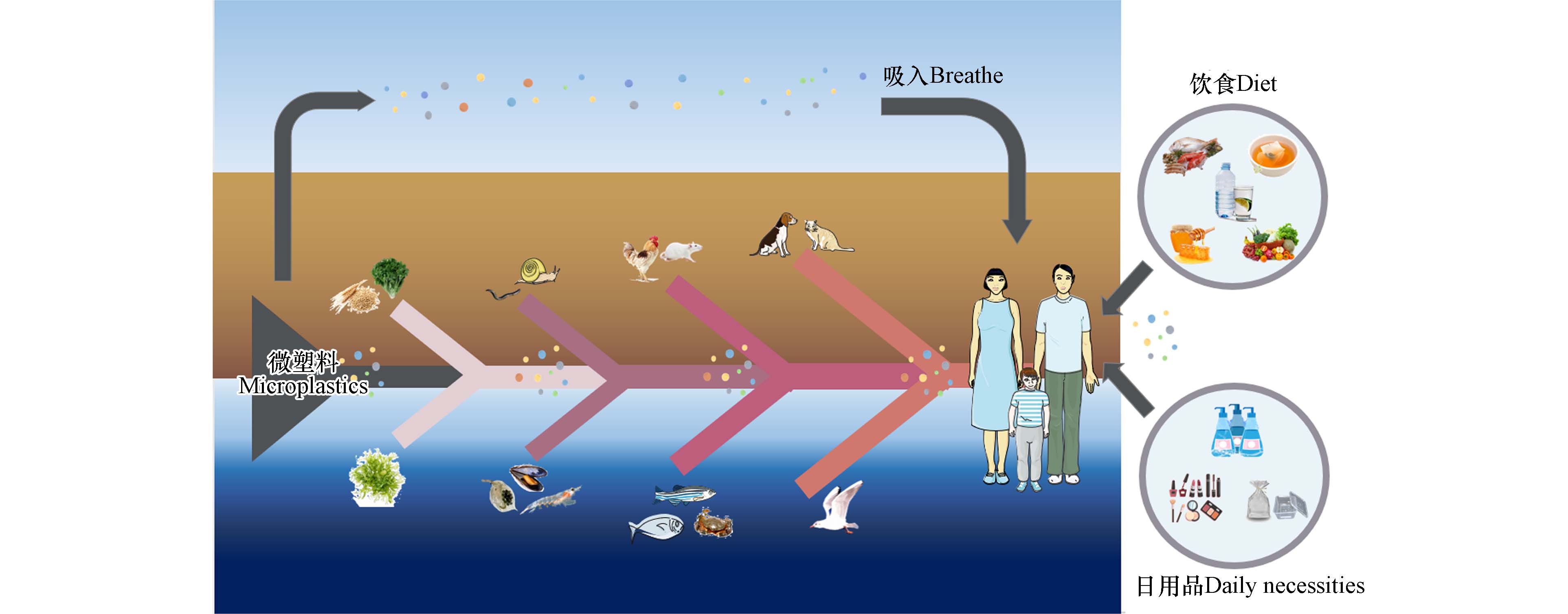
1000 nm、可以表现出胶体行为的塑料颗粒.自然界生物几乎完全暴露于广泛存在的微塑料污染之中[15],且由于微塑料自身难降解的特性[16],这种暴露将会是一种持久性暴露状态. 微塑料可伴随食物、水或空气通过消化道、呼吸道和皮肤接触3种途径进入生物体内[17],目前已在人类皮肤[18]、粪便[19]、胎盘[20]、血液(血栓)[21]等样本中检出了微塑料,证实了人类已直接暴露于微塑料,且婴幼儿和未成年人暴露于微塑料的健康风险可能更高[20].
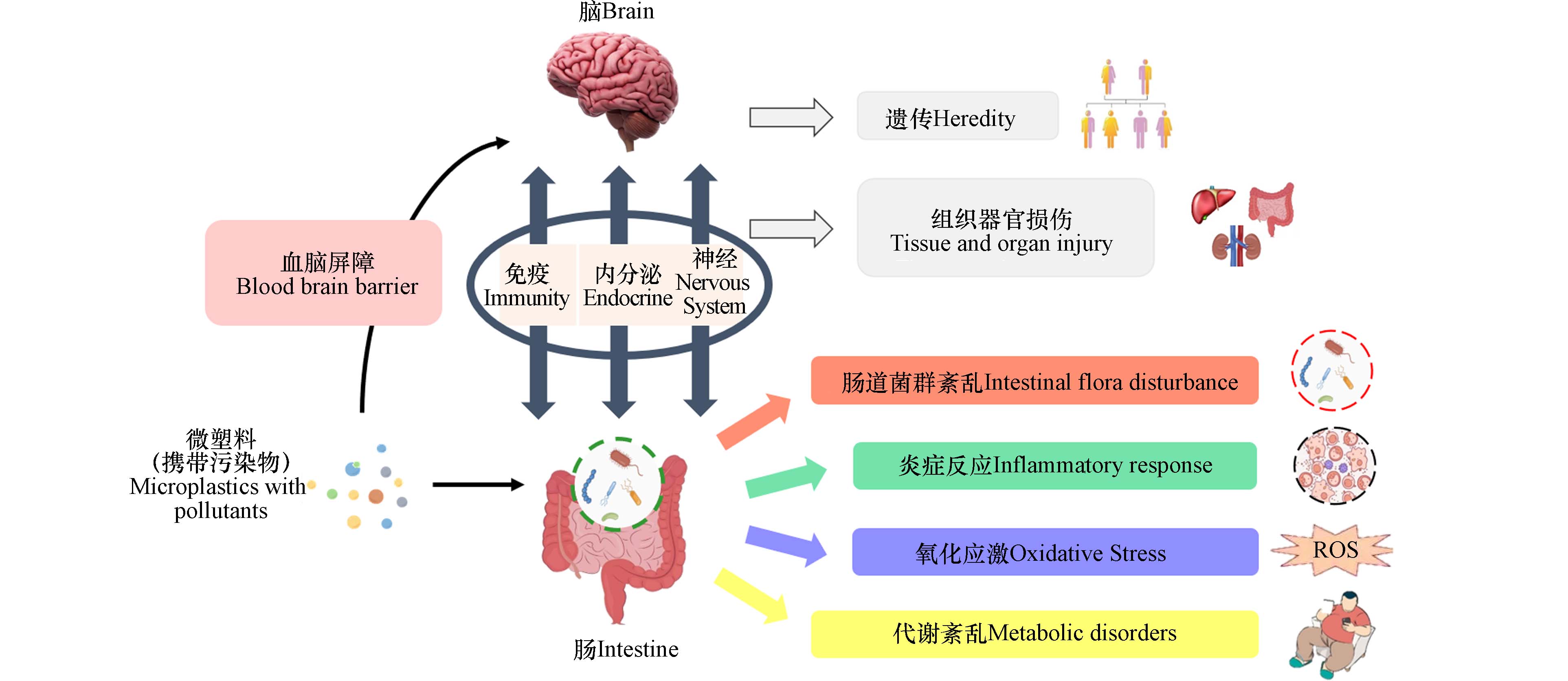
生物体的微塑料暴露虽不会引起急性死亡,但能够引起机体一系列不良反应,如氧化应激、炎症和免疫系统损伤、代谢紊乱、组织器官损伤、细胞毒性、DNA损伤与遗传毒性等,甚至可诱导人类机体产生癌变[17]. 有证据表明,微塑料暴露可能参与了炎症性肠病、癌症或肿瘤的发生或诱导[19]. 另外,较小尺寸的微塑料和纳米塑料更有可能直接通过肠道进入血液循环,从而导致更加严重的生物体危害[22]. 微塑料除自身对机体带来毒性外,还具有能够吸附、携带其他污染物与毒物的能力,并能够通过食物链向上累积[23],如图1所示.
-
胃肠道主要发挥消化和吸收营养物质的作用,作为生物体与外部环境接触的主要途径之一,很容易受到来自体外的潜在威胁[24]. 大脑能够通过中枢神经系统(central nervous system, CNS)、自主神经系统(autonomic nervous system,ANS)、肠神经系统(enteric nervous system,ENS)和下丘脑-垂体-肾上腺轴(hypothalamic pituitary gonadal axis,HPA)等神经内分泌通路与胃肠道进行双向交流,这一复杂的信号系统被称为“肠-脑轴”(Gut-Brain Axis)[25]. 这一概念最早由美国哥伦比亚大学神经学家迈克·格尔松教授提出,并将由肠管、肠道神经系统、肠道微生物形成的系统称为人体的“第二大脑”[26]. 目前认为包括交感神经和副交感神经在内的自主神经系统在“肠-脑轴”中发挥主要作用,肠道、脊髓和迷走神经组成了核心的交流通路[24].
越来越多的证据表明,肠道微生物群在肠道与大脑的双向交流中发挥重要作用[27],并与抑郁症[28]、帕金森病、阿尔茨海默病、亨廷顿病[25]、精神分裂症[29]、自闭症[30]、物质成瘾[31]等疾病的发生、发展有关. Cryan等[27,32]将其扩展为“微生物-肠-脑轴”,以描述胃肠道微生物与中枢神经系统之间的双向交流,并认为外在环境如饮食、药物等都能够调节肠道菌群,进而通过MGB轴对大脑及行为产生影响. 研究表明,环境中广泛存在的重金属锰、铅、镉、砷等能够通过胃肠道吸收暴露影响MGB轴[33]. 燃烧物产生的衍生纳米颗粒暴露于人体后大量存在于红细胞、无髓鞘粘膜、血管周围和肌内神经纤维、神经节神经元和迷走神经中,构成了帕金森病和阿尔茨海默病发病机制的可能途径[34]. 李文豪等[35]通过Spearman相关系数分析发现高原低氧环境能够导致小鼠肠道菌群结构改变,且艾克曼菌属、另枝菌属和瘤胃球菌属相对丰度的增加与小鼠学习记忆能力下降相关. 韦思佳等[36]通过粪菌移植改善了低菌鼠的学习记忆能力. 这些研究均表明,在肠脑交流过程中,肠道菌群可能发挥了重要的作用. 目前的研究认为,肠道菌群与大脑至少可以通过免疫、内分泌、神经三条通路及其交互作用进行交流[37].
-
人是一个“超级生物体”[38],体内有极其丰富的微生物群落. 人体肠道微生物重量近乎肝脏[32],被称为“人体第二基因组”. 肠道微生物包括酵母、古菌、寄生虫等,但最具特征的是细菌[27]. 肠道菌群与宿主健康密切相关. 研究表明,肠道菌群参与了人体消化和营养过程[39],可通过引发内毒素血症、改变短链脂肪酸信号以及胆碱和胆汁酸代谢等多种途径影响宿主代谢,而宿主代谢紊乱又能反作用于肠道微生物使其组成发生变化[40]. 肠道菌群作为肠道中的一个重要组成部分,不仅与肠道粘膜构成“微生物屏障”阻止病原菌入侵[39],还参与了肠道免疫过程,并与免疫系统外的多个器官构成了“肠-心轴”[41] “肠-肝轴”[42] “肠-肺轴”[43] “肠-脾轴”[44] “肠-肾轴”[45] “肠-脑轴”[46] “肠-肌轴”[47] “肠-骨轴”[48]等通路. 正常情况下, 肠道菌群与宿主间存在着复杂而微妙的动态平衡[49],一旦这种平衡被打破发生肠道菌群失调,可诱导某些疾病的发生. 如图2所示,研究表明肠道菌群失衡与炎症性肠病[50]、肠易激综合征、营养不良[51]、肥胖[52]、糖尿病、心脑血管疾病[53]、慢性肝病[54]、慢性肾病[55]、抑郁症[56]、阿尔茨海默症、自闭症[57]、神经性厌食症[58]、过敏、类风湿关节炎、癌症[59]等多种疾病的发生、发展相关[60]. 饮食是最容易被改变或控制的显著影响肠道菌群组成的环境因素之一[40,61],同样也是环境污染物生物暴露的主要途径之一. 因此,肠道菌群也被视为环境污染物的重要毒理学靶点. 近来的研究表明,除了重金属、大气污染物、持久性有机污染物、抗生素、纳米材料颗粒等[62],微塑料也可引起肠道菌群的紊乱.
微塑料暴露可改变生物肠道菌群组成与多样性,且这种影响在短期内即可显现. Xie等[63]通过7 d的短期实验发现,MPs暴露使小鼠肠道菌群结构和组成比例发生变化:在门水平上,聚丙烯组与肥胖密切相关的Firmicutes/Bacteroidetes比值迅速升高;在科水平上,聚苯乙烯组与炎症性肠炎的发生发展有关的Ruminococcaceae和Lachnospiraceae相对丰度显著增加;在属水平上,聚苯乙烯组可以保护宿主免受结肠炎的细菌Alistipes相对丰度显著下降. 较长时间暴露的研究也获得同样实验结果,Liu等[64]发现聚苯乙烯微塑料暴露(5 μm,40 mg·L−1,21 d)使中华绒螯蟹肠道菌群组成中的 Firmicutes 和 Bacteroidetes 的相对丰度下降,Fusobacteria 和Proteobacteria 的相对丰度上升. Wang等[65]以鲤鱼为模型的研究结果同样显示聚苯乙烯暴露后,鲤鱼肠道微生物群中Lactococcus garvieae,Bacteroides_paurosaccharolyticus,Romboutsia_ilealis的相对丰度显著降低,16S Silva数据库分析结果显示发生了与传染病、癌症、神经退行性疾病、内分泌和代谢性疾病、心血管疾病等疾病相关的肠道菌群紊乱. 以肠道免疫失衡小鼠为模型的研究发现,聚苯乙烯微塑料暴露不仅对结肠微生物群落和代谢产生了更严重的干扰,而且引起了类杆菌等致病菌数量的增加[66].
由于环境中的微塑料与实验室模拟实验使用的原始微塑料在性质上有所不同,Hu等[67]通过碾磨农用薄膜模拟次级聚乙烯微塑料,用其喂养鲫鱼30 d后,Alpha多样性指数显示实验组鲫鱼的肠道微生物群多样性显著增加,Firmicutes相对丰度显著升高,Fusobacteria和Bacteroidetes则降低,同时在细菌组成成分中发现了有害菌种. Zhang等[68]进一步研究了老化聚苯乙烯单独或与100 μg·L−1罗红霉素联合作用对鲫鱼肠道菌群的影响,测序结果显示联合暴露增加了Gemmobacter、Bosea、Rhizobium和Shinella的丰度,降低了Cetbacterium和Akkermansia的丰度.
通常微塑料的毒性与粒径显著相关[69],因此许多学者将纳米塑料与微塑料区别开来比较二者对肠道菌群的不同毒性效应. Xie等[70]比较了聚苯乙烯微米与纳米塑料(8 μm、80 nm,0.01 mg·L−1、1 mg·L−1)暴露 21 d对斑马鱼肠道菌群的影响,结果表明二者均可诱导斑马鱼肠道菌群失调,放线菌的丰度在 NPs 处理组中增加而在 MPs 处理组中下降;NPs 暴露增加了肠道中 IL8、IL10、IL1β和 TNF-α的表达,而在 MPs 暴露组中没有观察到相同情况. Li等[71]研究了淡水河蚌暴露于聚苯乙烯塑料(2 μm、80 nm,0.1 mg·L−1、1 mg·L−1)10 d后的肠道损伤情况和肠道菌群变化,发现二者都能诱导炎症反应,引起肠道上皮损伤,并能显著改变肠道微生物群落结构,但组间存在差异表达基因:MPs 更多地诱导先天免疫反应,激活补体和凝血级联途径(补体系统),引起的微生物紊乱更严重;NPs 优先诱导与细胞成分相关的过程,并通过线粒体途径触发组织(特别是间接接触组织)的凋亡,引起的粘膜损伤更严重. Kang等[72]将海洋青鳉暴露于聚苯乙烯(50 nm、45 μm)后,比较发现 NPs 暴露组表现出更强的氧化应激;MPs 暴露组表现出更严重的肠道损伤诱导,肠道菌群在门、属水平上表现出更显著的组成变化. 这些研究结果表明,微塑料和纳米塑料暴露都能够引起生物肠道菌群的紊乱与炎症反应,但由于微塑料具有更大的表面积,因此微塑料暴露对肠道菌群的紊乱影响更显著,且更多地诱导了免疫功能障碍;而纳米塑料则更显著地诱导氧化应激与细胞凋亡.
目前较多的研究是以实验动物为模型开展,少量研究涉及了微塑料暴露对人体肠道菌群的影响探究. Fournier等[73]研究发现,MPs/NPs环境暴露影响婴儿肠道菌群种类组成,使菌群中有益菌群丰度减少而有害菌群丰度增加,且这一变化受到暴露微塑料尺寸、浓度和种类的影响. Zhang等[74]的研究首次报告了长期暴露于微塑料环境对人体微生物的扰动影响,通过比较微塑料高暴露人群(在成都某塑料厂周围工作生活的20名受试者,环境塑料检出点
1050 个)与低暴露人群(在成都环花溪公园周围工作生活的20名受试者,环境塑料检出点46个)的肠道微生物发现,高暴露可能会增加与疾病有关的微生物丰度而减少有益微生物的丰度,并可能改变肠道微生物群与鼻腔微生物的共生关系. 这一报告以人群为研究对象,填补了关于微塑料暴露对生物体肠道菌群影响研究的空白,为人类健康研究提供了直接证据,但目前类似的研究仍较少. 微塑料暴露对生物肠道菌群影响的总结见表1. -
越来越多的研究表明,微塑料有能力突破血脑屏障进入生物大脑,并引起神经毒性反应. Shosaku[79]、Karin等[80]及Ding等[81]分别将青鳉、黑鲫、尼罗罗非鱼暴露在原始或经氨基修饰的PS-NPs中,不仅在实验动物的肠道、鳃、肝脏中检出了微塑料,在脑组织中也检出塑料颗粒,值得注意的是,研究结果表明仅暴露一周后,微塑料即进入了生物大脑,且伴随脑重量减轻、行为改变及脑回增大、乙酰胆碱酯酶活性被抑制、超氧化物歧化酶含量增加等表现. 对于哺乳纲动物,Jin等[82]研究发现,BALB/c小鼠连续180 d饮用含有PS-MPs(0.5 μm、4 μm、10 μm,100 μg·L−1、
1000 μg·L−1)的水后,PS-MPs在小鼠大脑中积累,出现血脑屏障破坏、树突棘密度降低、海马区炎症等反应,并出现了认知和记忆缺陷. Deng等[83]研究同样发现,PS-MPs能在肝脏、肾脏和肠道中积累,并使神经递质水平发生变化. Xu等[84]通过口服灌胃使小鼠暴露在微塑料中28 d,发现原始、氨基修饰(NH2—)和羧基修饰(COOH—)的PS-NPs(100 nm,1 mg·d−1)均能在肾脏、睾丸、脾脏、肺、胃肠道和大脑等器官中蓄积. 另外关于Wistar大鼠模型的研究表明,大鼠连续35 d摄入PS-NPs(25 nm、50 nm,1—10 mg·(kg·d)−1)不会引起体重的显著变化,其行为学表现也仅显示出微小的差异,虽然这一研究没有揭示显著的差异,但Rafiee等[85]认为这种微小差异同样具有临床意义. Liu等[86]的研究表明,PS-NPs(80 nm)可以通过雾化吸入的方式到达并沉积在小鼠大脑中,小鼠的活动表现水平下降,且较小的PS-NPs可诱导更多的细胞摄取. Chu等[87]将小鼠暴露于25 nm PS后发现,PS-NPs暴露增加了氧化应激和DNA损伤,严重损伤突触功能. Wang等[88]的研究表明PS-MPs暴露通过诱导氧化应激和降低乙酰胆碱水平进而影响学习和记忆. Ban等[89]利用神经细胞—人神经母细胞瘤细胞SH-SY5Y研究PS-NPs的细胞毒性,结果表明PS暴露促进细胞分化为神经元表型,神经突触生长收缩,细胞核形态改变,细胞内成分溢出. Shan等[90]发现,PS-NPs对小鼠大脑的小胶质细胞产生暴露后诱导了细胞活化和神经元损伤. Hua等[91]利用三维模型模拟人类大脑皮层发育研究微塑料暴露对人脑的影响,结果表明微塑料长期暴露能够降低神经细胞的活力.此外,微塑料对环境其他有毒污染物具有吸附、富集和载体效应,能够通过浓缩、增加接触和生物有效性、干扰机体防御等机制增加其他污染物毒性[92]. Barboza等[93]的研究表明,微塑料增加了欧洲鲈鱼幼鱼鳃和肝脏中汞的富集量,且诱导鳃和肝脏器官发生氧化应激. 安浩等[94]通过探究微塑料和三氯生联合暴露对斑马鱼的神经毒性效应,发现联合暴露使超氧化物歧化酶水平、乙酰胆碱酯酶活力降低,5-HT含量提高,并且诱导了98个特异性变化的代谢物,涉及氨基酸代谢、甘油磷脂代谢、鞘脂代谢、花生四烯酸代谢和神经活性配体受体相互作用等代谢过程,对脑产生更严重的神经毒性. Liu等[95]发现,微塑料与铁联合暴露可能通过扰乱脑组织中铁稳态及诱导认知相关区域的铁中毒而加重认知障碍.
微塑料不仅能够通过个体直接的饮食或呼吸暴露引起神经毒性,还可通过食物链的向上传递或子代传递间接引起神经毒性. da Costa Araújo等[96]的研究表明,小鼠通过食物链摄入微塑料后,在其肝脏中检出塑料颗粒,且小鼠出现缺乏防御性的社会聚集、风险评估行为减少等神经心理行为状态改变的表现. 这表明微塑料被摄食后可以通过食物链向更高营养级传递,且生物营养链级的水平越高,微塑料对生物体的毒性作用就越复杂[23]. Chen等[97]关于秀丽隐杆线虫的研究表明,PS-MPs(1.002 ± 0.005 μm,10 — 100 μg·L−1)暴露能够显著减少亲代及后代线虫的头部摆动及身体弯曲频率,且母体暴露于高浓度(100 μg·L)PS-MPs时,子代活性氧与脂褐素的显著增多,氧化应激相关基因(clk-1,ctl-1,sod-3,sod-4,sod-5)表达上调. 这表明微塑料暴露引起的神经毒性可实现跨代表现,且氧化应激在其中起着重要作用. 实验研究证实,对于微藻[98]、涡虫 [99]、线虫[100]、鲤鱼 [101]、斑马鱼[75]、小鼠[69]等不同物种及大鼠卵巢颗粒细胞[102]、L929小鼠成纤维细胞、狗肾上皮细胞株[103]、人血管内皮细胞 [104]、人结直肠腺癌细胞[103]、人正常肺上皮细胞[105]等不同类型的细胞,微塑料均引起了不同程度的氧化应激.
-
生物暴露于低剂量微塑料时首先通过免疫反应来适应污染环境,而高剂量的微塑料阻碍了这一平衡的恢复,最终导致了抑制或毒性作用,表现出炎症. Liu等[65]发现PS-MPs 暴露使中华绒螯蟹血淋巴和肝胰脏中的大多数免疫相关因子的含量(活性)随着暴露剂量的增加和时间的延长表现出“先增加、后下降”的趋势,并引起了 Hc、LSZ、MyD88 和 caspase 等免疫基因表达的改变. Li等[76]、Jin等[78]的研究结果显示,MPs暴露使小鼠肠道粘液分泌减少,肠道屏障功能损伤,TLR4、AP-1和IRF5表达增加,高浓度MPs通过激活TLR4信号通路诱发了小肠炎症. Liu等[66]比较了0.50 mg·L−1 PS-MPs对健康小鼠和肠道免疫失衡小鼠的影响,PS-MPs暴露可显著增加肠道免疫失衡小鼠炎症因子的表达,加重肠道免疫失衡小鼠结肠黏膜的组织病理损伤. 由于自身肠道免疫失衡的个体对环境污染会更加敏感,因此在今后的健康风险研究与评估中必须考量这一问题.
微塑料对生物内分泌系统造成干扰. Chen等[106]的测定结果显示,在野外采集的海洋微塑料中,双酚A的检出频率最高,其次是双酚S、辛基酚和壬基酚,还包括雌激素等,这些内分泌干扰物来自于环境吸附或制造添加. Ullah等[107]认为双酚、邻苯二甲酸盐、多溴联苯醚、多氯联苯醚、有机锡、全氟化合物等容易经微纳塑料转运,哺乳动物接触后内分泌系统将受到影响,且较小的颗粒能释放出更多的内分泌干扰物. Lin等[108]的结果显示,PSMPs的存在增加了斑马鱼性腺中微囊藻毒素(microcystin-LR,MC-LR)的积累,增强了MC-LR诱导的生殖毒性,与17β-雌二醇和睾酮水平的异常升高密切相关. Chenet等[109]通过量化评估方法发现,接受微塑料暴露后60%的大西洋马鲛肝组织中有肝脏卵黄蛋白原表达,证实摄入微塑料可能会改变内分泌系统功能.
微塑料生物暴露将引起机体代谢紊乱. Fan等[110]研究发现PS-MPs使微藻氧化胁迫和毒素产生增加,改变了微藻代谢谱,且较大尺寸和较高浓度的PS-MPs诱导了大量差异表达的代谢物,显著干扰参与氨基酸合成、膜形成、氮素储存和抗氧化防御的代谢途径. Zhong等[111]通过测定摄食量、甘油三酯、血糖、活动量、雌性产卵量等分析PA-MPs对果蝇的影响,结果表明食用含有微塑料的食物会导致氧化应激和炎症,从而影响果蝇的营养代谢. Xie等[63]通过7 d的短期实验拟合各小鼠的体重增长曲线发现,MPs暴露,特别是PET与PP,对小鼠体重增长的贡献显著. Li等[76]、Jin等[78]通过KEGG分析以及氨基酸代谢和胆汁酸代谢指标结果证实了PS-MPs暴露引起了小鼠代谢紊乱. Deng等[83]的研究发现,PS-MPs能在肝脏、肾脏和肠道中积累,引起能量和脂质代谢紊乱. Xu等[84]通过口服灌胃使小鼠暴露在原始、氨基修饰或羧基修饰的PS-NPs(100 nm,1 mg·d−1)28 d后,发现NPs导致组织学损伤、血液系统损伤及脂代谢紊乱.
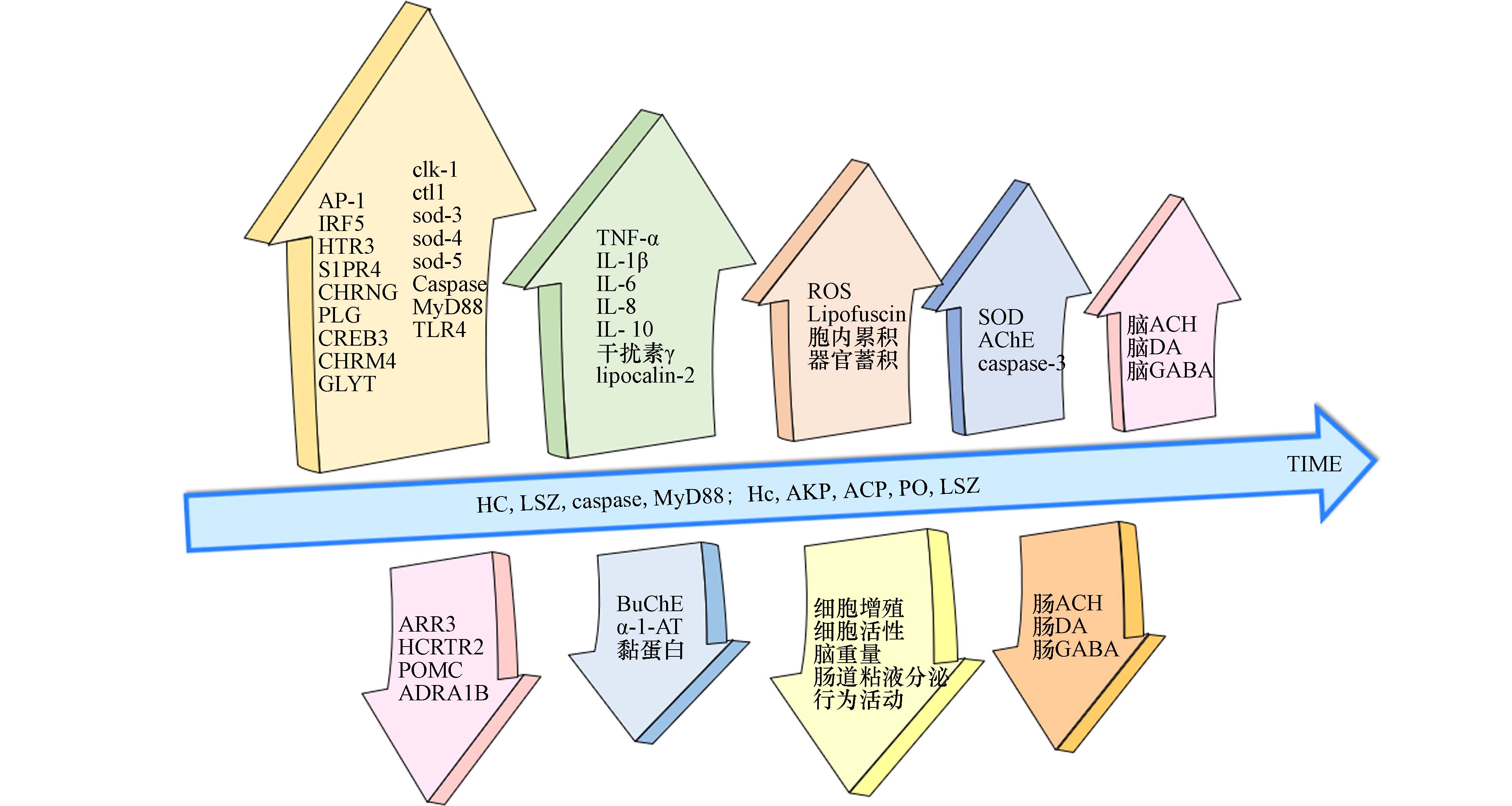
关于微塑料暴露对MGB轴的影响,目前的研究很少,且缺乏机制阐释. Huang等[112]将铁饼鱼暴露在不同浓度(20 μg·L−1、200 μg·L−1)的PA超细纤维(400 ø × 900 µm)和PS-NPs(约 88 ø nm)中96 h后发现,铁饼鱼的捕食和游泳能力下降;鱼脑中的乙酰胆碱酯酶被激活而丁酰胆碱酯酶被抑制;大脑中的神经递质(乙酰胆碱、多巴胺和γ-氨基丁酸)浓度有所升高,而肠道中则普遍降低;肠道菌群结构有所改变,变形菌门比例在NPs组降低而在MPs组增加,梭杆菌属比例均降低;脑转录组显示与神经活动和递质受体相关的7个基因(HTR3、S1PR4、CHRNG、PLG、CREB3、CHRM4、GLYT)表达上调,4个基因(ARR3、HCRTR2、POMC、ADRA1B)表达下调,KEGG通路富集分析显示MPs组和NPs组均与神经活性配体-受体相互作用通路及血清素能突触通路相关,多巴胺能突触通路仅与MPs组相关. Teng等[113]的研究显示,斑马鱼持续30 d暴露在PS-NPs(44 nm,1 μg·L−1、10 μg·L−1、100 μg·L−1)环境后,生长受到抑制,出现炎症反应;靶向代谢组学分析揭示了42种代谢物(包括神经递质)的改变,且有14种代谢物的变化与3个微生物类群(变形菌门、厚壁菌门、拟杆菌门)的变化相关. 微塑料暴露引起的部分基因表达与生化因子水平变化见图3. 这些研究均表明,微塑料,特别是纳米塑料,对生物形成暴露后,不仅能够引起肠道菌群紊乱和神经毒性对生物健康产生威胁,并且极有可能通过“肠道菌群—肠—脑轴”这一通路进行交流. 虽然现有关于微塑料暴露引起肠道菌群失衡、引发炎症与代谢紊乱、干扰内分泌系统和神经系统等生物毒性效应的研究很多且较为深入,已经涉及机制探讨方面. 但现有研究的内容相对独立,缺乏更加系统、全面的讨论,特别是关注微塑料对MGB轴系统通路的影响与机制研究的报道很少,目前仍处于起步阶段,且研究缺乏对高等哺乳动物的关注,大量研究结论的得出更多来自于实验室模拟研究而非实证研究. 由于当宿主肠道菌群失衡时,可通过外源添加益生菌[114]、粪便菌群移植[115 − 117]等方式对肠道菌群进行调节,从而起到改善不良反应或疾病表现的作用,因此开展关于微塑料暴露对“肠道菌群-肠-脑轴”影响和作用机制的深入研究,对于预防微塑料污染威胁、保护生物健康具有重要意义.
-
本文从微塑料肠道菌群毒性、神经毒性及“肠道菌群-肠-脑轴”机制等方面综述了国内外关于微塑料生物暴露的研究. 由于目前去除、回收和降解微塑料的技术不足以实现环境中微塑料的完全消除,因此由环境微塑料生物暴露导致的机体健康风险问题势必需要引起足够的重视. 微塑料能够通过消化道、呼吸道、皮肤等途径进入生物体内,单独或与其他环境污染物联合暴露直接引起肠道菌群紊乱和神经毒性(如行为改变、可能的神经发育障碍等),并有可能通过“肠道菌群-肠-脑轴”介导毒性效应. 目前有关微塑料暴露通过“肠道菌群-肠-脑轴”引起神经毒性,特别是在哺乳动物相关表现研究及机理探索方面才刚刚起步,未来的研究工作重点可关注以下方面:
(1)微塑料生物暴露的神经毒性效应与机制研究. 目前有研究证据表明微塑料特别是纳米塑料能够突破血脑屏障进入生物大脑,或通过影响内分泌、免疫、氧化应激水平等途径间接引起神经毒性效应,然而当前研究模拟的暴露条件与真实环境相差较大,存在微塑料种类单一、染毒剂量偏高、模拟暴露时间较短等不足,且对微塑料暴露引起神经毒性的机制探索尚不完全清晰,因此未来对这一方面的研究需更加深入开展.
(2)微塑料暴露对高等动物特别是暴露人群的潜在毒性效应与机制研究. 当前在微塑料生物毒性研究中使用的实验模型多基于水生生物、啮齿动物、人源细胞等,为相关领域探索奠定了研究基础、提供了大量证据,然而对于高等动物、特别是人群而言,微塑料进入机体内后可能引起更为复杂或潜在的毒性效应,目前关于微塑料在生物体内迁移、吸收、代谢的具体机制也尚不清楚,因此需要更加关注微塑料暴露对高等动物及人群的影响及机制探究.
(3)微塑料暴露对“肠道菌群-肠-脑轴”毒性及作用机制的研究. 大量研究表明,微塑料暴露能够引起机体肠道菌群失调,进而导致一系列毒性效应. 而“肠道菌群-肠-脑轴”作为不同机体系统交流的重要途径,在维持生物健康方面发挥着重要的生理作用. “肠道菌群-肠-脑轴”既是微塑料暴露对机体产生不良影响的重要途径之一,也是寻求预防和抵抗微塑料暴露风险的潜在作用点之一,而目前国内外关于此方面的探索才刚刚开展,仍然存在大量研究空白. 因此,关于微塑料暴露对“肠道菌群-肠-脑轴”的毒性影响,特别是有关作用机制的研究需进一步开展.
微塑料暴露引起“肠道菌群-肠-脑轴”毒性及作用机制研究进展
Research progress on toxicity and mechanism of gut microbiota-gut-brain axis induced by microplastics and nanoplastics exposure
-
摘要: 微塑料由于其自身粒径小、吸附性强、难降解等特点,不但在自然界广泛存在,而且可通过消化道、呼吸道、皮肤接触等途径使得生物长期暴露其污染之中,并通过食物链不断向上累积. 有研究发现,微塑料单独或与其他污染物联合暴露能够对机体产生如肠道菌群紊乱、神经行为毒性等不良影响. 肠道菌群在许多生理过程中发挥着重要作用,并可能通过“肠道菌群-肠-脑轴”与大脑进行双向交流,这一路径与许多神经、精神疾病的发生及发展相关. 目前关于微塑料对“肠道菌群-肠-脑轴”的毒性影响及作用机制的研究较少. 本文综述了近几年国内外关于微塑料毒性及“肠道菌群-肠-脑轴”的研究,为进一步探讨微塑料对肠道菌群及神经行为的毒性效应、研究潜在的毒性机制、寻找可能存在的生物标志物与防护措施、保障环境生态与人类健康提供研究思路.Abstract: Microplastics have the characteristics of small particle size, strong adsorption, difficulty in degradation, etc. Therefore, microplastics are not only widely present in nature, but also cause long-term pollution to organisms through pathways such as digestive tract, respiratory tract, and skin contact, and can continuously accumulate upwards through the food chain. Studies have found that exposure to microplastics alone or in combination with other pollutants can have adverse effects on the body, such as disruptions in gut microbiota and neurobehavioral toxicity. The gut microbiota plays an important role in many physiological processes and may engage in bi-directional communications with the brain through the microbiota-gut-brain axis(MGBA), which is associated with the occurrence and development of many neurological and psychiatric diseases. So far, few studies have examined the toxic effects and underlying mechanisms of microplastics on the MGBA. This review summarized the latest literature on the toxicity of microplastics and the MGBA, in order to stimulate new research ideas on 1) the toxic effects of microplastics on microbiota and neurobehavior, 2) the potential mechanisms underlying such effects, 3) identifying possible biomarkers and preventive measures, and 4) protecting the environment and human health.
-
Key words:
- microplastics /
- toxicity /
- gut microbiota-gut-brain axis /
- mechanisms.
-

-
表 1 微/纳米塑料暴露引起肠道菌群紊乱
Table 1. Disruption of intestinal flora caused by Micro/Nano-plastics
实验对象 微/纳米塑料暴露 菌群结构改变 文献 Research object Characteristics of MPs/NPs Change of microbial community References 中华绒螯蟹 PS(5 μm,40 mg·L−1,21 d) ↓ Firmicutes、Bacteroidetes
↑ Fusobacteria、Proteobacteria[64] 斑马鱼 PS(5 μm,0.05 、0.50 mg·L−1,21 d) ↓ Proteobacteria
↑ Fusobacteria[75] 斑马鱼 PS(8 μm,0.01、1 mg·L−1,21 d) ↓ Fusobacteria,Firmicutes、Verrucomicrobiota、Actinomycetes [70] ↑ Proteobacteria PS(80 nm,0.01、1 mg·L−1,21 d) ↓ Fusobacteria,Firmicutes、Verrucomicrobiota ↑ Proteobacteria、Actinomycetes 鲤鱼 PS(8 μm,0.08 mg·L−1,21 d) ↓ Lactococcus garvieae,Bacteroides_paurosaccharolyticus,Romboutsia_ilealis
↑ Plesiomonas shigelloides[65] 鲫鱼 PE(<848.37 μm,6.38、12.18、
22.33 mg·(MPs·fish·d)−1,30 d)↓ Fusobacteria,Bacteroidetes [67] ↑ Firmicutes 鲫鱼 PS-ROX (5 μm ,100 ug·L−1,28 d) ↓ Cetbacterium,Akkermansia [68] ↑ Gemmobacter,Bosea、Rhizobium、Shinella 昆明小鼠 PS(150 — 300 µm,4 mg·L−1,7 d) ↓ Alistipes
↑ Ruminococcaceae、Lachnospiraceae[63] PP(150 — 300 µm,4 mg·L−1,7 d) ↓ Bacteroidetes
↑ FirmicutesC57BL/6小鼠 PE(10 — 150 µm,6、60、
600 ug·L−1,5 w)↓ Bacteroidetes、Bacterodides Muribaculum、unidentified Clostridiales、Akkermansia
↑ Firmicutes、melainabacobacteria、Blautia、Desulfovibrio[76] ICR小鼠 PE(1 — 10 µm,0.002、
0.2 ug·(g·d)−1,15 d)↓ Firmicutes
↑ Bacteroides[77] ICR小鼠 PS(5 µm,100、 1000 µg·L−1,6 w)↓ Firmicutes、Proteobacteria、 Actinobacteria、Bacteroidetes
↑ Coprococcus、Anaeroplasma[78] 人群 PU、SR、ACR等25种塑料 ↓ Bacteroides、Ruminococcus、Dorea、Fusobacterium、Coprococcus
↑ Klebsiella、Helicobacter、Bifidobacterium、Streptococcus、Sphingomonas[74] 注:聚苯乙烯(polystyrene,PS),聚乙烯(polyethylene,PE),聚丙烯(polypropylene,PP),聚氨酯(polyurethane,PU),硅树脂(silicon resin,SR),丙烯酸树脂(acrylic resin,ACR),罗红霉素(Roxithromycin,ROX). ↑表示菌群相对丰度增加,↓表示菌群相对丰度减少. ↑indicates that the relative abundance of bacteria increases, and↓ indicates that the relative abundance of bacteria decreases. -
[1] DERRAIK J G B. The pollution of the marine environment by plastic debris: A review[J]. Marine Pollution Bulletin, 2002, 44(9): 842-852. doi: 10.1016/S0025-326X(02)00220-5 [2] MATO Y, ISOBE T, TAKADA H, et al. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment[J]. Environmental Science & Technology, 2001, 35(2): 318-324. [3] THOMPSON R C, OLSEN Y, MITCHELL R P, et al. Lost at sea: Where is all the plastic?[J]. Science, 2004, 304(5672): 838. doi: 10.1126/science.1094559 [4] WONG J K H, LEE K K, TANG K H D, et al. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions[J]. Science of the Total Environment, 2020, 719: 137512. doi: 10.1016/j.scitotenv.2020.137512 [5] LI W X, LI X, TONG J, et al. Effects of environmental and anthropogenic factors on the distribution and abundance of microplastics in freshwater ecosystems[J]. The Science of the Total Environment, 2023, 856(Pt 2): 159030. [6] XU H T, LI L A, WANG Y J, et al. Differential physiological response of marine and freshwater microalgae to polystyrene microplastics[J]. Journal of Hazardous Materials, 2023, 448: 130814. doi: 10.1016/j.jhazmat.2023.130814 [7] NIZZETTO L, FUTTER M, LANGAAS S. Are agricultural soils dumps for microplastics of urban origin?[J]. Environmental Science & Technology, 2016, 50(20): 10777-10779. [8] 骆永明, 周倩, 章海波, 等. 重视土壤中微塑料污染研究 防范生态与食物链风险[J]. 中国科学院院刊, 2018, 33(10): 1021-1030. LUO Y M, ZHOU Q, ZHANG H B, et al. Pay attention to research on microplastic pollution in soil for prevention of ecological and food chain risks[J]. Bulletin of Chinese Academy of Sciences, 2018, 33(10): 1021-1030 (in Chinese).
[9] 范姿, 卞倩. 气载微塑料对呼吸系统的影响及机制研究进展[J]. 环境化学, 2023, 42(6): 1792-1802. doi: 10.7524/j.issn.0254-6108.2022011301 FAN Z, BIAN Q. Research progress on the effects and mechanisms of airborne microplastics exposure on the respiratory system[J]. Environmental Chemistry, 2023, 42(6): 1792-1802 (in Chinese). doi: 10.7524/j.issn.0254-6108.2022011301
[10] ABAD LÓPEZ A P, TRILLERAS J, ARANA V A, et al. Atmospheric microplastics: Exposure, toxicity, and detrimental health effects[J]. RSC Advances, 2023, 13(11): 7468-7489. doi: 10.1039/D2RA07098G [11] WANG W F, GE J, YU X Y, et al. Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective[J]. The Science of the Total Environment, 2020, 708: 134841. doi: 10.1016/j.scitotenv.2019.134841 [12] AJITH N, ARUMUGAM S, PARTHASARATHY S, et al. Global distribution of microplastics and its impact on marine environment-a review[J]. Environmental Science and Pollution Research International, 2020, 27(21): 25970-25986. doi: 10.1007/s11356-020-09015-5 [13] ANDRADY A L. Microplastics in the marine environment[J]. Marine Pollution Bulletin, 2011, 62(8): 1596-1605. doi: 10.1016/j.marpolbul.2011.05.030 [14] GIGAULT J, TER HALLE A, BAUDRIMONT M, et al. Current opinion: What is a nanoplastic?[J]. Environmental Pollution, 2018, 235: 1030-1034. doi: 10.1016/j.envpol.2018.01.024 [15] COX K D, COVERNTON G A, DAVIES H L, et al. Human consumption of microplastics[J]. Environmental Science & Technology, 2019, 53(12): 7068-7074. [16] CHEN J L, WU J, SHERRELL P C, et al. How to build a microplastics-free environment: Strategies for microplastics degradation and plastics recycling[J]. Advanced Science, 2022, 9(6): e2103764. doi: 10.1002/advs.202103764 [17] KUMAR R, MANNA C, PADHA S, et al. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans?[J]. Chemosphere, 2022, 298: 134267. doi: 10.1016/j.chemosphere.2022.134267 [18] ABBASI S, TURNER A. Human exposure to microplastics: A study in Iran[J]. Journal of Hazardous Materials, 2021, 403: 123799. doi: 10.1016/j.jhazmat.2020.123799 [19] YAN Z H, LIU Y F, ZHANG T, et al. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status[J]. Environmental Science & Technology, 2022, 56(1): 414-421. [20] RAGUSA A, SVELATO A, SANTACROCE C, et al. Plasticenta: First evidence of microplastics in human placenta[J]. Environment International, 2021, 146: 106274. doi: 10.1016/j.envint.2020.106274 [21] WU D, FENG Y D, WANG R, et al. Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence[J]. Journal of Advanced Research, 2023, 49: 141-150. doi: 10.1016/j.jare.2022.09.004 [22] MORTENSEN N P, FENNELL T R, JOHNSON L M. Unintended human ingestion of nanoplastics and small microplastics through drinking water, beverages, and food sources[J]. NanoImpact, 2021, 21: 100302. doi: 10.1016/j.impact.2021.100302 [23] TOUSSAINT B, RAFFAEL B, ANGERS-LOUSTAU A, et al. Review of micro- and nanoplastic contamination in the food chain[J]. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2019, 36(5): 639-673. [24] GRODZICKI W, DZIENDZIKOWSKA K, GROMADZKA-OSTROWSKA J, et al. Nanoplastic impact on the gut-brain axis: Current knowledge and future directions[J]. International Journal of Molecular Sciences, 2021, 22(23): 12795. doi: 10.3390/ijms222312795 [25] 李兆清, 任泽盛, 李彤昕, 等. 微生物-肠-脑轴与神经退行性疾病的关系[J]. 神经解剖学杂志, 2022, 38(5): 593-597. LI Z Q, REN Z S, LI T X, et al. Relationships between microbial-gut-brain axis and neurodegenerative diseases[J]. Chinese Journal of Neuroanatomy, 2022, 38(5): 593-597 (in Chinese).
[26] 邓琦蕾, 申元英. 肠道微生物群在脑-肠-微生物轴中作用机制的研究进展[J]. 实用医学杂志, 2017, 33(14): 2404-2407. DENG Q L, SHEN Y Y. Research progress on the mechanism of intestinal microflora in brain-intestine-microorganism axis[J]. The Journal of Practical Medicine, 2017, 33(14): 2404-2407 (in Chinese).
[27] CRYAN J F, O'RIORDAN K J, COWAN C S M, et al. The microbiota-gut-brain axis[J]. Physiological Reviews, 2019, 99(4): 1877-2013. doi: 10.1152/physrev.00018.2018 [28] 刘兰香, 王海洋, 谢鹏. 肠道微生物紊乱诱导抑郁的肠-脑分子机制研究[J]. 重庆医科大学学报, 2021, 46(9): 1003-1007. LIU L X, WANG H Y, XIE P. Study of gut microbiota dysbiosis induced depression and the underlying gut-brain mechanisms[J]. Journal of Chongqing Medical University, 2021, 46(9): 1003-1007 (in Chinese).
[29] 全晨阳, 柴剑波, 赵永厚. 微生物-肠-脑轴与精神分裂症相关性研究进展[J]. 中华中医药杂志, 2022, 37(9): 5284-5287. QUAN C Y, CHAI J B, ZHAO Y H. Review on the correlation researches between microbial-gut-brain axis and schizophrenia[J]. China Journal of Traditional Chinese Medicine and Pharmacy, 2022, 37(9): 5284-5287 (in Chinese).
[30] YU Y, ZHAO F Q. Microbiota-gut-brain axis in autism spectrum disorder[J]. Journal of Genetics and Genomics, 2021, 48(9): 755-762. doi: 10.1016/j.jgg.2021.07.001 [31] 来思敏, 王彪, 王婧, 等. 肠道菌群与物质成瘾的关系及研究进展[J]. 西安交通大学学报(医学版), 2023, 44(6): 841-851. LAI S M, WANG B, WANG J, et al. Relationship between gut microbiota and substance addiction and its research progress[J]. Journal of Xi'an Jiaotong University(Medical Sciences), 2023,44(6):841-851(in Chinese).
[32] GE T T, YAO X X, ZHAO H S, et al. Gut microbiota and neuropsychiatric disorders: Implications for neuroendocrine-immune regulation[J]. Pharmacological Research, 2021, 173: 105909. doi: 10.1016/j.phrs.2021.105909 [33] 侯艳文, 库婷婷, 桑楠. 重金属影响中枢神经系统的微生物-肠-脑轴途径[J]. 环境化学, 2019, 38(3): 454-462. doi: 10.7524/j.issn.0254-6108.2018050501 HOU Y W, KU T T, SANG N. Influences of heavy metal on the central nervous system through microbe-gut-brain axis pathway[J]. Environmental Chemistry, 2019, 38(3): 454-462 (in Chinese). doi: 10.7524/j.issn.0254-6108.2018050501
[34] CALDERÓN-GARCIDUEÑAS L, REYNOSO-ROBLES R, PÉREZ-GUILLÉ B, et al. Combustion-derived nanoparticles, the neuroenteric system, cervical vagus, hyperphosphorylated alpha synuclein and tau in young Mexico City residents[J]. Environmental Research, 2017, 159: 186-201. doi: 10.1016/j.envres.2017.08.008 [35] 李文豪, 齐宝宁, 施艺, 等. 模拟高原低氧小鼠学习记忆能力与肠道菌群结构改变的相关性研究[J]. 中国临床解剖学杂志, 2023, 41(3): 296-303. LI W H, QI B N, SHI Y, et al. Correlation between learning and memory ability and gut microbiota structure in mice induced by high-altitude hypoxia[J]. Chinese Journal of Clinical Anatomy, 2023, 41(3): 296-303 (in Chinese).
[36] 韦思佳, 许永劼, 杨婷婷, 等. 探讨低菌鼠认知功能的改变及粪菌移植的改善作用[J]. 现代预防医学, 2023, 50(1): 145-150. WEI S J, XU Y J, YANG T T, et al. Low-level bacterium rats’ cognitive function and its improvement by fecal bacteria transplantation[J]. Modern Preventive Medicine, 2023, 50(1): 145-150 (in Chinese).
[37] AGIRMAN G, HSIAO E Y. SnapShot: The microbiota-gut-brain axis[J]. Cell, 2021, 184(9): 2524-2524. e1. [38] LEDERBERG J. Infectious history[J]. Science, 2000, 288(5464): 287-293. doi: 10.1126/science.288.5464.287 [39] 汪洪涛. 肠道微生物与人体健康的关系及其影响因素研究进展[J]. 食品安全质量检测学报, 2022, 13(1): 175-181. WANG H T. Research progress on the relationship between intestinal microorganisms and human health and its influencing factors[J]. Journal of Food Safety & Quality, 2022, 13(1): 175-181 (in Chinese). influencing factors[J]. Journal of Food Safety & Quality, 2022, 13(1): 175–181(in Chinese).
[40] JANSSEN A W F, KERSTEN S. The role of the gut microbiota in metabolic health[J]. FASEB Journal:Official Publication of the Federation of American Societies for Experimental Biology, 2015, 29(8): 3111-3123. doi: 10.1096/fj.14-269514 [41] ZHAO J X, ZHANG Q, CHENG W, et al. Heart-gut microbiota communication determines the severity of cardiac injury after myocardial ischaemia/reperfusion[J]. Cardiovascular Research, 2023, 119(6): 1390-1402. doi: 10.1093/cvr/cvad023 [42] HAMOUD A R, WEAVER L, STEC D E, et al. Bilirubin in the liver-gut signaling axis[J]. Trends in Endocrinology & Metabolism, 2018, 29(3): 140-150. [43] BINGULA R, FILAIRE M, RADOSEVIC-ROBIN N, et al. Desired turbulence?gut-lung axis, immunity, and lung cancer[J]. Journal of Oncology, 2017, 2017: 5035371. [44] BARREA L, Di SOMMA C, MUSCOGIURI G, et al. Nutrition, inflammation and liver-spleen axis[J]. Critical Reviews in Food Science and Nutrition, 2018, 58(18): 3141-3158. doi: 10.1080/10408398.2017.1353479 [45] ROSSI M, JOHNSON D W, CAMPBELL K L. The kidney-gut axis: Implications for nutrition care[J]. Journal of Renal Nutrition:the Official Journal of the Council on Renal Nutrition of the National Kidney Foundation, 2015, 25(5): 399-403. doi: 10.1053/j.jrn.2015.01.017 [46] KEIGHTLEY P C, KOLOSKI N A, TALLEY N J. Pathways in gut-brain communication: Evidence for distinct gut-to-brain and brain-to-gut syndromes[J]. The Australian and New Zealand Journal of Psychiatry, 2015, 49(3): 207-214. doi: 10.1177/0004867415569801 [47] LIAO X S, WU M T, HAO Y T, et al. Exploring the preventive effect and mechanism of senile sarcopenia based on gut-muscle axis[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 590869. doi: 10.3389/fbioe.2020.590869 [48] ZAISS M M, JONES R M, SCHETT G, et al. The gut-bone axis: How bacterial metabolites bridge the distance[J]. The Journal of Clinical Investigation, 2019, 129(8): 3018-3028. doi: 10.1172/JCI128521 [49] 林璋, 祖先鹏, 谢海胜, 等. 肠道菌群与人体疾病发病机制的研究进展[J]. 药学学报, 2016, 51(6): 843-852. LIN Z, ZU X P, XIE H S, et al. Research progress in mechanism of intestinal microorganisms in human diseases[J]. Acta Pharmaceutica Sinica, 2016, 51(6): 843-852 (in Chinese).
[50] WU G D, BUSHMANC F D, LEWIS J D. Diet, the human gut microbiota, and IBD[J]. Anaerobe, 2013, 24: 117-120. doi: 10.1016/j.anaerobe.2013.03.011 [51] BARRATT M J, AHMED T, GORDON J I. Gut microbiome development and childhood undernutrition[J]. Cell Host & Microbe, 2022, 30(5): 617-626. [52] SANCHEZ M, PANAHI S, TREMBLAY A. Childhood obesity: A role for gut microbiota?[J]. International Journal of Environmental Research and Public Health, 2014, 12(1): 162-175. doi: 10.3390/ijerph120100162 [53] ZOU X L, WANG L Y, XIAO L X, et al. Gut microbes in cerebrovascular diseases: Gut flora imbalance, potential impact mechanisms and promising treatment strategies[J]. Frontiers in Immunology, 2022, 13: 975921. doi: 10.3389/fimmu.2022.975921 [54] LEUNG D H, YIMLAMAI D. The intestinal microbiome and paediatric liver disease[J]. The Lancet Gastroenterology & Hepatology, 2017, 2(6): 446-455. [55] MAFRA D, FOUQUE D. Gut microbiota and inflammation in chronic kidney disease patients[J]. Clinical Kidney Journal, 2015, 8(3): 332-334. doi: 10.1093/ckj/sfv026 [56] 段佳佳. 肠道微生物动态演变与小鼠抑郁状态及抗抑郁药疗效的机制研究[D]. 重庆: 重庆医科大学, 2021. DUAN J J. Study on the mechanism between dynamic alteration of gut microbiome with depression and antidepressant efficacy in mice[D]. Chongqing: Chongqing Medical University, 2021 (in Chinese).
[57] DUQUE A L R F, DEMARQUI F M, SANTONI M M, et al. Effect of probiotic, prebiotic, and synbiotic on the gut microbiota of autistic children using an in vitro gut microbiome model[J]. Food Research International, 2021, 149: 110657. doi: 10.1016/j.foodres.2021.110657 [58] TRINH S, KELLER L, SEITZ J. The gut microbiome and its clinical implications in anorexia nervosa[J]. Zeitschrift Fur Kinder- Und Jugendpsychiatrie Und Psychotherapie, 2021, 50(3): 227-237. [59] 安瑞, 赵丰, 吴盛海, 等. 人肠道菌群与肺癌关系的研究进展[J]. 中国微生态学杂志, 2022, 34(1): 113-116,125. AN R, ZHAO F, WU S H, et al. Progress in research on the relationship between human gut microbes and lung cancer[J]. Chinese Journal of Microecology, 2022, 34(1): 113-116,125 (in Chinese).
[60] 郭慧玲, 邵玉宇, 孟和毕力格, 等. 肠道菌群与疾病关系的研究进展[J]. 微生物学通报, 2015, 42(2): 400-410. GUO H L, SHAO Y Y, MENG H, et al. Research on the relation between gastrointestinal microbiota and disease[J]. Microbiology China, 2015, 42(2): 400-410 (in Chinese).
[61] 翟齐啸, 田丰伟, 王刚, 等. 肠道微生物与人体健康的研究进展[J]. 食品科学, 2013, 34(15): 337-341. ZHAI Q X, TIAN F W, WANG G, et al. Progress in research on the role of intestinal microbiota in human health[J]. Food Science, 2013, 34(15): 337-341 (in Chinese).
[62] 丁晗, 周童, 王娟, 等. 典型环境污染物对肠道菌群的影响及机制研究进展[J]. 生态毒理学报, 2021, 16(2): 34-49. DING H, ZHOU T, WANG J, et al. Research progress on the effects of typical environmental pollutants on gut microbiota and their underlying mechanisms[J]. Asian Journal of Ecotoxicology, 2021, 16(2): 34-49 (in Chinese).
[63] XIE L L, CHEN T L, LIU J Y, et al. Intestinal flora variation reflects the short-term damage of microplastic to the intestinal tract in mice[J]. Ecotoxicology and Environmental Safety, 2022, 246: 114194. doi: 10.1016/j.ecoenv.2022.114194 [64] LIU Z Q, YU P, CAI M Q, et al. Effects of microplastics on the innate immunity and intestinal microflora of juvenile Eriocheir sinensis[J]. The Science of the Total Environment, 2019, 685: 836-846. doi: 10.1016/j.scitotenv.2019.06.265 [65] WANG F H, ZHANG Q R, CUI J, et al. Polystyrene microplastics induce endoplasmic reticulum stress, apoptosis and inflammation by disrupting the gut microbiota in carp intestines[J]. Environmental Pollution, 2023, 323: 121233. doi: 10.1016/j.envpol.2023.121233 [66] LIU S, LI H, WANG J, et al. Polystyrene microplastics aggravate inflammatory damage in mice with intestinal immune imbalance[J]. Science of the Total Environment, 2022, 833: 155198. doi: 10.1016/j.scitotenv.2022.155198 [67] HU J M, ZUO J E, LI J B, et al. Effects of secondary polyethylene microplastic exposure on crucian (Carassius carassius) growth, liver damage, and gut microbiome composition[J]. The Science of the Total Environment, 2022, 802: 149736. doi: 10.1016/j.scitotenv.2021.149736 [68] ZHANG P, LU G H, SUN Y, et al. Metagenomic analysis explores the interaction of aged microplastics and roxithromycin on gut microbiota and antibiotic resistance genes of Carassius auratus[J]. Journal of Hazardous Materials, 2022, 425: 127773. doi: 10.1016/j.jhazmat.2021.127773 [69] ZOU H, QU H Y, BIAN Y S, et al. Polystyrene microplastics induce oxidative stress in mouse hepatocytes in relation to their size[J]. International Journal of Molecular Sciences, 2023, 24(8): 7382. doi: 10.3390/ijms24087382 [70] XIE S L, ZHOU A G, WEI T L, et al. Nanoplastics induce more serious microbiota dysbiosis and inflammation in the gut of adult zebrafish than microplastics[J]. Bulletin of Environmental Contamination and Toxicology, 2021, 107(4): 640-650. doi: 10.1007/s00128-021-03348-8 [71] LI Z L, FENG C H, PANG W, et al. Nanoplastic-induced genotoxicity and intestinal damage in freshwater benthic clams (Corbicula fluminea): Comparison with microplastics[J]. ACS Nano, 2021, 15(6): 9469-9481. doi: 10.1021/acsnano.1c02407 [72] KANG H M, BYEON E, JEONG H, et al. Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka Oryzias melastigma[J]. Journal of Hazardous Materials, 2021, 405: 124207. doi: 10.1016/j.jhazmat.2020.124207 [73] FOURNIER E, RATEL J, DENIS S, et al. Exposure to polyethylene microplastics alters immature gut microbiome in an infant in vitro gut model[J]. Journal of Hazardous Materials, 2023, 443(Pt B): 130383. [74] ZHANG X Y, WANG H T, PENG S H, et al. Effect of microplastics on nasal and intestinal microbiota of the high-exposure population[J]. Frontiers in Public Health, 2022, 10: 1005535. doi: 10.3389/fpubh.2022.1005535 [75] QIAO R X, SHENG C, LU Y F, et al. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish[J]. Science of the Total Environment, 2019, 662: 246-253. doi: 10.1016/j.scitotenv.2019.01.245 [76] LI B Q, DING Y F, CHENG X, et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice[J]. Chemosphere, 2020, 244: 125492. doi: 10.1016/j.chemosphere.2019.125492 [77] SUN H Q, CHEN N, YANG X N, et al. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice[J]. Ecotoxicology and Environmental Safety, 2021, 220: 112340. doi: 10.1016/j.ecoenv.2021.112340 [78] JIN Y X, LU L, TU W Q, et al. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice[J]. Science of the Total Environment, 2019, 649: 308-317. doi: 10.1016/j.scitotenv.2018.08.353 [79] KASHIWADA S. Distribution of nanoparticles in the see-through medaka (Oryzias latipes)[J]. Environmental Health Perspectives, 2006, 114(11): 1697-1702. doi: 10.1289/ehp.9209 [80] MATTSSON K, JOHNSON E V, MALMENDAL A, et al. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain[J]. Scientific Reports, 2017, 7: 11452. doi: 10.1038/s41598-017-10813-0 [81] DING J N, ZHANG S S, RAZANAJATOVO R M, et al. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus)[J]. Environmental Pollution, 2018, 238: 1-9. doi: 10.1016/j.envpol.2018.03.001 [82] JIN H B, YANG C, JIANG C Y, et al. Evaluation of neurotoxicity in BALB/c mice following chronic exposure to polystyrene microplastics[J]. Environmental Health Perspectives, 2022, 130(10): 107002. doi: 10.1289/EHP10255 [83] DENG Y F, ZHANG Y, LEMOS B, et al. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure[J]. Scientific Reports, 2017, 7: 46687. doi: 10.1038/srep46687 [84] XU D H, MA Y H, HAN X D, et al. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells[J]. Journal of Hazardous Materials, 2021, 417: 126092. doi: 10.1016/j.jhazmat.2021.126092 [85] RAFIEE M, DARGAHI L, ESLAMI A, et al. Neurobehavioral assessment of rats exposed to pristine polystyrene nanoplastics upon oral exposure[J]. Chemosphere, 2018, 193: 745-753. doi: 10.1016/j.chemosphere.2017.11.076 [86] LIU X Y, ZHAO Y C, DOU J B, et al. Bioeffects of inhaled nanoplastics on neurons and alteration of animal behaviors through deposition in the brain[J]. Nano Letters, 2022, 22(3): 1091-1099. doi: 10.1021/acs.nanolett.1c04184 [87] CHU C, ZHANG Y L, LIU Q P, et al. Identification of ceRNA network to explain the mechanism of cognitive dysfunctions induced by PS NPs in mice[J]. Ecotoxicology and Environmental Safety, 2022, 241: 113785. doi: 10.1016/j.ecoenv.2022.113785 [88] WANG S W, HAN Q, WEI Z L, et al. Polystyrene microplastics affect learning and memory in mice by inducing oxidative stress and decreasing the level of acetylcholine[J]. Food and Chemical Toxicology, 2022, 162: 112904. doi: 10.1016/j.fct.2022.112904 [89] BAN M, SHIMODA R, CHEN J. Investigation of nanoplastic cytotoxicity using SH-SY5Y human neuroblastoma cells and polystyrene nanoparticles[J]. Toxicology in Vitro:an International Journal Published in Association With BIBRA, 2021, 76: 105225. [90] SHAN S, ZHANG Y F, ZHAO H W, et al. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice[J]. Chemosphere, 2022, 298: 134261. doi: 10.1016/j.chemosphere.2022.134261 [91] HUA T, KIRAN S, LI Y, et al. Microplastics exposure affects neural development of human pluripotent stem cell-derived cortical spheroids[J]. Journal of Hazardous Materials, 2022, 435: 128884. doi: 10.1016/j.jhazmat.2022.128884 [92] 赵美静, 夏斌, 朱琳, 等. 微塑料与有毒污染物相互作用及联合毒性作用研究进展[J]. 生态毒理学报, 2021, 16(5): 168-185. ZHAO M J, XIA B, ZHU L, et al. Research progress on interaction and joint toxicity of microplastics with toxic pollutants[J]. Asian Journal of Ecotoxicology, 2021, 16(5): 168-185 (in Chinese).
[93] BARBOZA L G A, VIEIRA L R, BRANCO V, et al. Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in Dicentrarchus labrax juveniles[J]. Scientific Reports, 2018, 8: 15655. doi: 10.1038/s41598-018-34125-z [94] 安浩, 张宴. 微塑料和三氯生对斑马鱼的神经毒性效应研究[J]. 能源环境保护, 2023, 37(4): 131-139. AN H, ZHANG Y. Study on neurotoxic effects of microplastics and triclosan on the zebrafish[J]. Energy Environmental Protection, 2023, 37(4): 131-139 (in Chinese).
[95] LIU X, YANG H K, YAN X Z, et al. Co-exposure of polystyrene microplastics and iron aggravates cognitive decline in aging mice via ferroptosis induction[J]. Ecotoxicology and Environmental Safety, 2022, 233: 113342. doi: 10.1016/j.ecoenv.2022.113342 [96] Da COSTA ARAÚJO A P, MALAFAIA G. Microplastic ingestion induces behavioral disorders in mice: A preliminary study on the trophic transfer effects via tadpoles and fish[J]. Journal of Hazardous Materials, 2021, 401: 123263. doi: 10.1016/j.jhazmat.2020.123263 [97] CHEN H B, HUA X, LI H, et al. Transgenerational neurotoxicity of polystyrene microplastics induced by oxidative stress in Caenorhabditis elegans[J]. Chemosphere, 2021, 272: 129642. doi: 10.1016/j.chemosphere.2021.129642 [98] ZHENG X W, ZHANG W Z, YUAN Y, et al. Growth inhibition, toxin production and oxidative stress caused by three microplastics in Microcystis aeruginosa[J]. Ecotoxicology and Environmental Safety, 2021, 208: 111575. doi: 10.1016/j.ecoenv.2020.111575 [99] HAN Y P, ZHANG X X, LIU P F, et al. Microplastics exposure causes oxidative stress and microbiota dysbiosis in planarian Dugesia japonica[J]. Environmental Science and Pollution Research International, 2022, 29(19): 28973-28983. doi: 10.1007/s11356-022-18547-x [100] YU Y J, CHEN H B, HUA X, et al. Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans[J]. The Science of the Total Environment, 2020, 726: 138679. doi: 10.1016/j.scitotenv.2020.138679 [101] XIA X H, SUN M H, ZHOU M, et al. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae[J]. Science of the Total Environment, 2020, 716: 136479. doi: 10.1016/j.scitotenv.2019.136479 [102] AN R, WANG X F, YANG L, et al. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats[J]. Toxicology, 2021, 449: 152665. doi: 10.1016/j.tox.2020.152665 [103] PALANIAPPAN S, SADACHARAN C M, ROSTAMA B. Polystyrene and polyethylene microplastics decrease cell viability and dysregulate inflammatory and oxidative stress markers of MDCK and L929 cells in vitro[J]. Exposure and Health, 2022, 14(1): 75-85. doi: 10.1007/s12403-021-00419-3 [104] CHEN Y C, CHEN K F, LIN K Y A, et al. Evaluation of toxicity of polystyrene microplastics under realistic exposure levels in human vascular endothelial EA. hy926 cells[J]. Chemosphere, 2023, 313: 137582. doi: 10.1016/j.chemosphere.2022.137582 [105] DONG C D, CHEN C W, CHEN Y C, et al. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment[J]. Journal of Hazardous Materials, 2020, 385: 121575. doi: 10.1016/j.jhazmat.2019.121575 [106] CHEN Q Q, ALLGEIER A, YIN D Q, et al. Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions[J]. Environment International, 2019, 130: 104938. doi: 10.1016/j.envint.2019.104938 [107] ULLAH S, AHMAD S, GUO X L, et al. A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals[J]. Frontiers in Endocrinology, 2023, 13: 1084236. doi: 10.3389/fendo.2022.1084236 [108] LIN W, LUO H M, WU J Y, et al. Polystyrene microplastics enhance the microcystin-LR-induced gonadal damage and reproductive endocrine disruption in zebrafish[J]. The Science of the Total Environment, 2023, 876: 162664. doi: 10.1016/j.scitotenv.2023.162664 [109] CHENET T, MANCIA A, BONO G, et al. Plastic ingestion by Atlantic horse mackerel (Trachurus trachurus) from central Mediterranean Sea: A potential cause for endocrine disruption[J]. Environmental Pollution, 2021, 284: 117449. doi: 10.1016/j.envpol.2021.117449 [110] FAN Y F, LIU T, QIAN X, et al. Metabolic impacts of polystyrene microplastics on the freshwater microalga Microcystis aeruginosa[J]. Science of the Total Environment, 2022, 836: 155655. doi: 10.1016/j.scitotenv.2022.155655 [111] ZHONG L C, JIN H, TANG H, et al. Intake of polyamide microplastics affects the behavior and metabolism of Drosophila[J]. Chemosphere, 2022, 308: 136485. doi: 10.1016/j.chemosphere.2022.136485 [112] HUANG J N, WEN B, XU L, et al. Micro/nano-plastics cause neurobehavioral toxicity in discus fish (Symphysodon aequifasciatus): Insight from brain-gut-microbiota axis[J]. Journal of Hazardous Materials, 2022, 421: 126830. doi: 10.1016/j.jhazmat.2021.126830 [113] TENG M M, ZHAO X L, WANG C J, et al. Polystyrene nanoplastics toxicity to zebrafish: Dysregulation of the brain-intestine-microbiota axis[J]. ACS Nano, 2022, 16(5): 8190-8204. doi: 10.1021/acsnano.2c01872 [114] 王涛, 田欣蕾, 张迪, 等. 益生菌调节肠道黏膜免疫研究进展[J]. 中国兽医学报, 2022, 42(12): 2578-2584. WANG T, TIAN X L, ZHANG D, et al. Research progress in probiotic bacteria modulating intestinal mucosal immunity[J]. Chinese Journal of Veterinary Science, 2022, 42(12): 2578-2584 (in Chinese).
[115] PARKER A, ROMANO S, ANSORGE R, et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain[J]. Microbiome, 2022, 10(1): 68. doi: 10.1186/s40168-022-01243-w [116] XIE Y Y, SONG L Y, YANG J H, et al. Small intestinal flora graft alters fecal flora, stool, cytokines and mood status in healthy mice[J]. Life Science Alliance, 2021, 4(9): e202101039. doi: 10.26508/lsa.202101039 [117] BAKKEN J S, BORODY T, BRANDT L J, et al. Treating Clostridium difficile infection with fecal microbiota transplantation[J]. Clinical Gastroenterology and Hepatology, 2011, 9(12): 1044-1049. doi: 10.1016/j.cgh.2011.08.014 -



 下载:
下载: