-
四环素(TC)是一种典型的广谱抗生素,广泛应用于人类疾病的防治和畜牧养殖业病害的预防,其在世界范围被大量使用,导致环境中残留的四环素越来越多[1-2],这些积累的四环素对人体健康和生态安全造成了严重危害[3-5]。因此水体中的TC污染去除受到人们越来越多的关注。
吸附法由于操作简单、吸附效率高等特点而备受关注,是一种去除水体中TC常用的方法[6-8]。吸附材料的孔道结构对分子尺寸大的污染物的吸附具有重要的影响,如孔道尺寸排斥效应的存在导致分子尺寸大的污染物极低的吸附[9,10]。通过扩孔或者调变孔径结构能增强分子尺寸大的有机物吸附性能,研究表明CO2活化制备的多孔炭,具有高孔隙体积和丰富的中孔,显著提高了对TC的吸附去除[11]。共价三嗪多孔聚合材料(CTFs)具有高的比表面积、有序的孔结构、良好的热稳定性和化学稳定性等特点[12-14],在气体储存和催化方面备受关注[15-17]。根据之前研究发现,CTFs对水体中有机污染如芳香族化合物苯、苯酚、苯胺和硝基苯等[18],以及双酚A[19]、腐殖酸[20]、染料等[21-24]具有很高的吸附性能,其具有大孔道能有效的避免尺寸排斥效应。因此,CTFs是一种吸附去除水体中TC潜在的吸附剂,但目前对于TC在CTFs的吸附机理,与CTFs孔结构作用关系的研究尚不清楚。
本研究采用离子热共聚法制备了两种孔径大小不同的CTFs材料,通过X射线衍射、红外光谱和N2吸附/脱附等方法对其结构和表面性质进行了表征。选取抗生素四环素(TC)作为模拟污染物,系统研究了CTFs对TC的吸附行为,包含等温线、动力学、pH值和离子强度的影响,揭示TC在CTFs上的吸附机理以及CTFs孔结构在吸附中的重要作用。
-
四环素、1,4-对苯二腈、4,4’-联苯甲腈购自Sigma-Aldrich公司。无水氯化锌、四氢呋喃、氯化钠、盐酸、氢氧化钠均为分析纯,购自南京化学试剂有限公司。
-
通过离子热共聚法合成CTFs[12]。无水氯化锌和1,4-对苯二腈按摩尔比1∶1在手套箱内研磨均匀,转移到石英管中,然后抽至真空至10−2 Pa,密封后加热至400 ℃保持40 h。反应结束后,将得到的材料碾磨,用1.0 mol·L−1盐酸溶液、蒸馏水、四氢呋喃洗涤数次,于150 ℃真空干燥12 h,得到CTF-1材料。
CTFDCBP合成:4,4’-联苯甲腈与无水氯化锌的摩尔比1:10,其他步骤同合成CTF-1。
-
X-射线衍射(XRD)分析利用日本Rigaku D/max-RAX射线衍射仪(Cu靶,Kα辐射源,扫描范围3°—50°);红外光谱使用美国Nexus 870光谱仪测定(KBr压片,扫描范围400—4000 cm−1);N2吸附/解吸等温线由美国Micrometrics ASAP2020孔径分析系统在−196℃条件下测定。
-
吸附实验在配有聚四氟乙烯盖子的玻璃瓶中(40 mL)进行,准确称取20 mg CTF-1和10 mg CTFDCBP,加入不同浓度的TC溶液(CTF-1浓度范围0.008—0.028 mol·L−1,CTFDCBP浓度范围0.080—0.353 mol·L−1)中,背景溶液为 0.02 mol·L−1 NaCl。样品用铝箔包好避光,室温条件下(22℃±2℃)旋转下吸附48 h,离心测定TC的浓度,实验采用两组平行实验。采用 Agilent1200高效液相色谱紫外检测器测定TC浓度,测定条件:波长360 nm,进样量0.1 mL,流动相0.01 mol·L−1草酸缓冲液、乙腈和甲醇(80:16:4,V∶V∶V),流速1.0 mL·min−1。
-
准确称取40 mg CTF-1和20 mg CTFDCBP分别加入到60 mL浓度为0.224 mol·L−1 TC溶液中。吸附平衡后测定液相浓度,用0.45 μm 微膜过滤收集吸附剂,40℃真空干燥48 h。吸附TC前后的吸附剂孔径分布变化情况采用N2吸附/解吸实验测定。
测定条件:空白吸附剂200℃活化8 h,吸附TC后的吸附剂80℃活化8 h(活化温度80℃是为了防止吸附的TC在高温下分解,从而影响孔径的测定结果)。
-
实验初始pH值用0.1 mol·L−1 HCl 和 NaOH 溶液调节至2至11之间(根据溶液pH计算加入酸碱的量),按前述吸附等温线方法进行实验,采用两组平行样,并测定吸附平衡时溶液的pH值。
-
选取背景液分别为 0.01、0.02、0.05、0.08、0.1 mol·L−1的氯化钠和氯化钙溶液,按前述吸附等温线方法进行吸附实验,采用两组平行样。
-
准确称取50 mg 吸附剂CTFDCBP于500 mL广口瓶中,加入初始浓度为0.224 mol·L−1 TC溶液500 mL,初始pH值为6.8 ± 0.2,温度为25 ℃,避光下进行吸附。磁力搅拌下于不同时间间隔取样,样品用 0.45 μm滤膜过滤,测定滤液中TC的浓度。
-
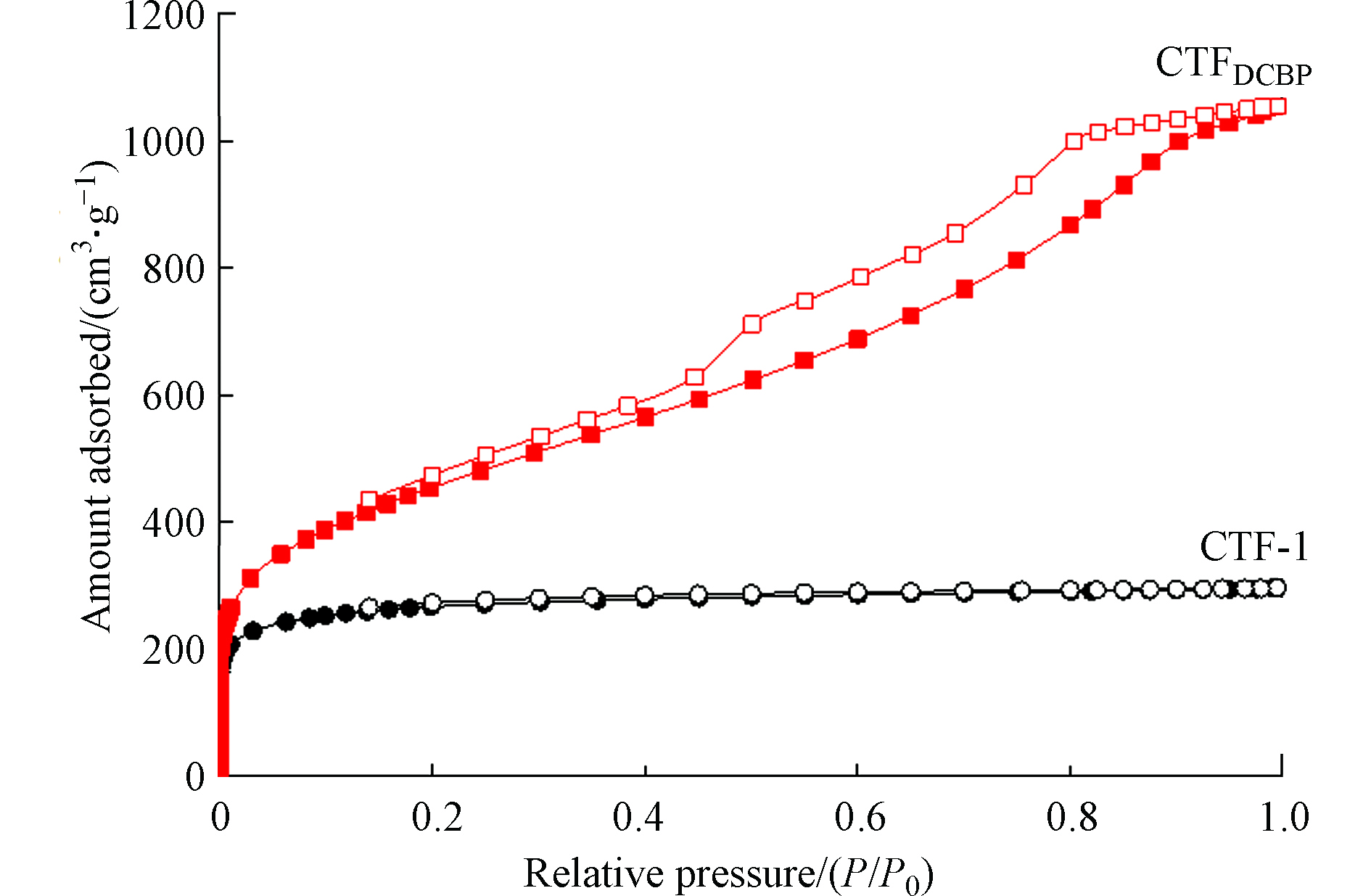
两种CTFs材料的N2吸附脱附等温线如图1所示,孔径分布参数列于表1。由图1可知,CTFDCBP在相对压力为0.20—0.90区间出现了滞后环,这是由于N2在CTFDCBP的孔道里发生了毛细凝聚,表明CTFDCBP具有中孔结构。这与表1的结果一致,CTFDCBP含有中孔结构,而CTF-1主要以微孔组成。CTFDCBP总孔体积为1.420 cm3·g−1,比表面积1745 m2·g−1,均高于CTF-1。
CTFs的XRD谱图如图2所示。从图2可知,CTF-1在2θ为7.2°、15.1°和26.2°处有3个峰,其中2θ在7.2°和15.1°处的峰为三嗪结构六角结构晶型的100晶面和200晶面,26.2°时的峰为芳香片层的001晶面[12]。而CTFDCBP仅存在着芳香片层结构的峰,说明CTFDCBP的孔道结构是无定形的,这主要是由于模板剂盐效应导致的,随着单体与氯化锌比例的增加,聚合物逐渐以无定形结构形式存在[14]。
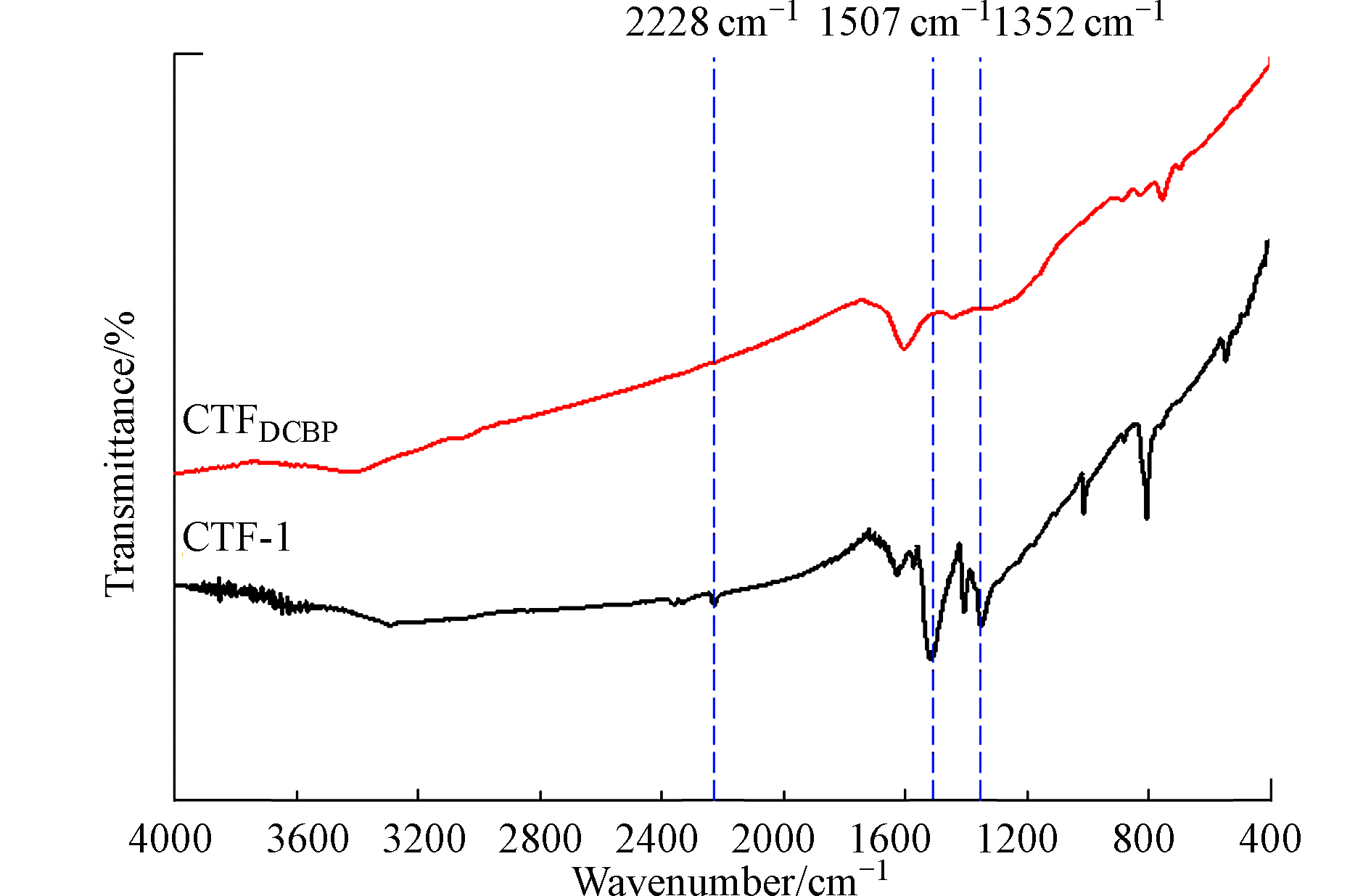
CTF-1和CTFDCBP的红外谱图如图3所示。从图3可以看出, CTF-1和CTFDCBP在1352、1507、2228 cm−1都有3个吸收峰,其中1507 cm−1和1352 cm−1归属于三嗪结构的伸缩振动和环呼吸振动,在2228 cm−1的峰归属于C≡N的伸缩振动[25]。
-
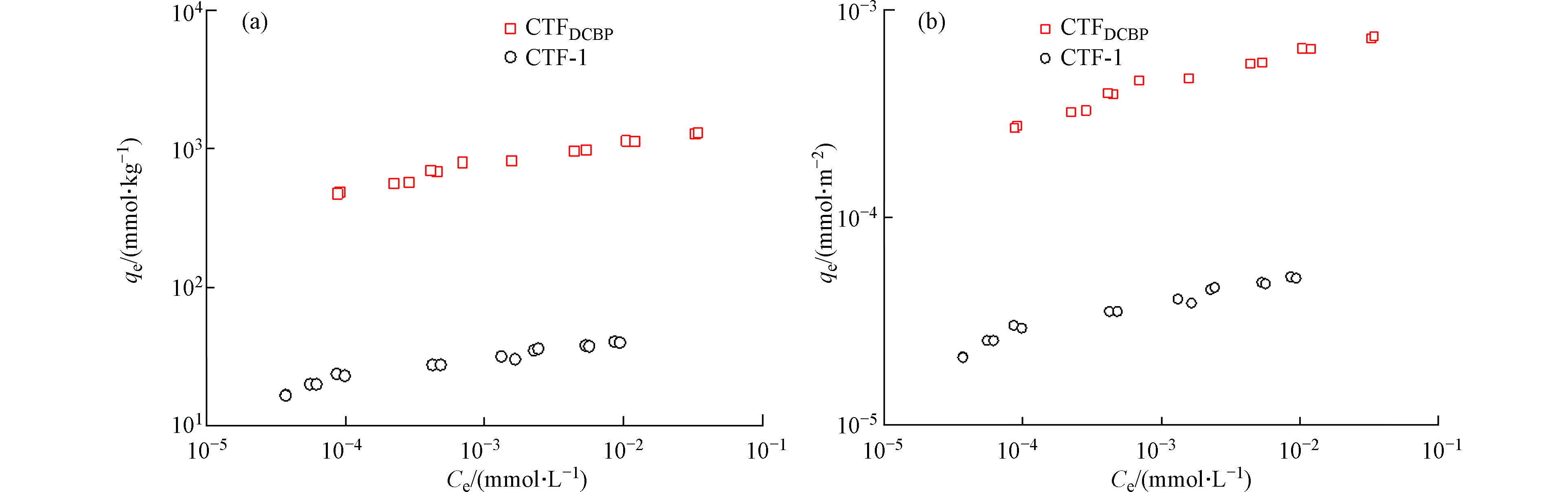
TC在两种CTFs上的吸附等温线如图4所示。采用Freundlich 吸附模型对吸附等温线拟合,拟合的参数列于表2。由表2可知,吸附数据拟合系数R2分别为0.9660和0.9800,表明Freundlich 吸附模型可以很好地对数据进行拟合。表2中吸附作用力常数n都小于1,表明吸附有很强的非线性,进一步说明吸附质与吸附剂的作用位点分布具有非均质性,主要是两者之间的特殊吸附作用或吸附剂本身具有较广的孔径分布范围所致。
由图4可知,两种CTFs对TC均有比较强的吸附作用。CTFs的空腔是由苯环和三嗪环混合组成,形成一个大的共轭链结构,具有丰富的π电子,可作为π电子供体。TC含有多种极性官能团如酚基、氨基、烯醇和酮基等,具有很强的吸电子能力,与其相连的芳香环或不饱和结构可以作为π电子受体,因此TC都可以与CTFs之间发生π-π电子交互作用[18]。此外,TC上质子化的氨基可能与CTFs上富π电子区域发生作用,形成阳离子-π 键[21]。这与Ji等[26,27]研究得到TC可与纳米碳管和石墨结构之间产生π-π电子和阳离子-π键作用机理是一致的。
对比图4a可知,TC在CTFDCBP的吸附量明显大于CTF-1。在实验浓度范围内,两种吸附剂的固相-液相的分配系数(Kd)分别为:CTF-1为103—105 L·kg−1,CTFDCBP为104—106 L·kg−1,CTFDCBP对TC的吸附亲和力比CTF-1高1—2个数量级。TC在两种CTFs之间的吸附亲和力差别是由于尺寸排斥效应造成的。TC分子尺寸大于微孔,不能进入CTF-1内部微孔结构中;而CTFDCBP具有中孔结构,有利于TC进入。比表面积标化(平衡吸附量除以吸附剂比表面积)后的吸附等温线可以更清晰显示出尺寸排斥效应对TC在两种CTFs上吸附亲和力的影响(图4b)。经过比表面积标化后,TC在两种CTFs材料上吸附亲和力仍然是CTFDCBP>CTF-1,这主要与TC能够接触利用的吸附剂表面积大小相关,CTFs吸附TC后孔径变化进一步说明孔径作用的影响。
-
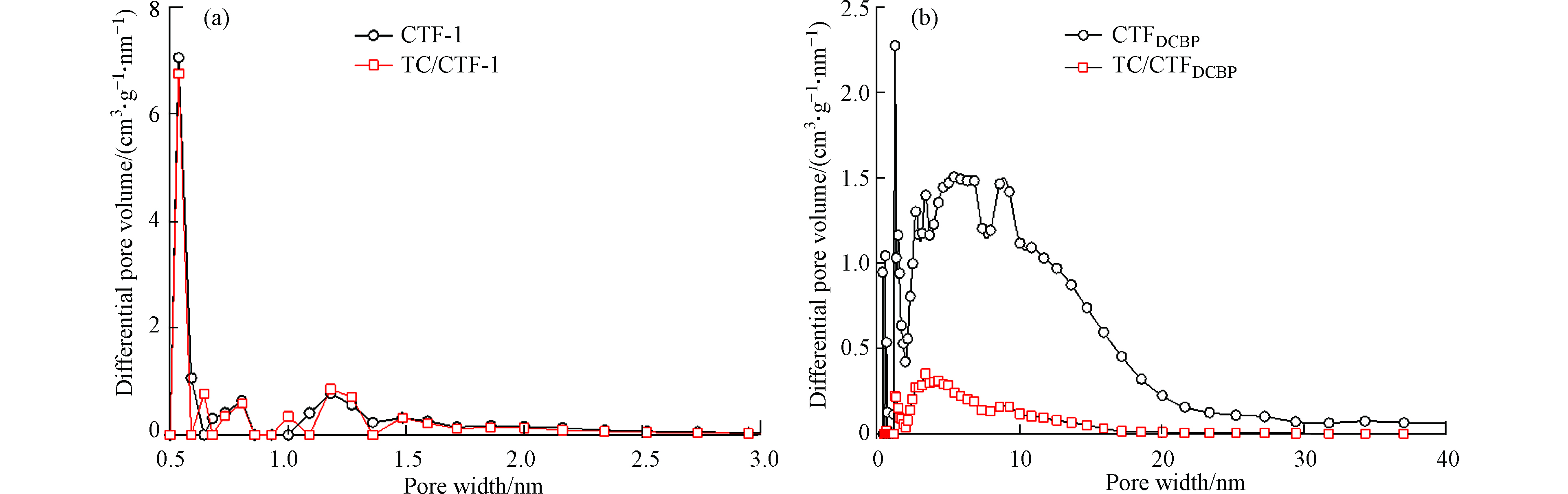
吸附TC后CTF-1和CTFDCBP比表面积分别是748.6 m2·g−1和220.5 m2·g−1,孔隙总体积分别为0.3920 cm3·g−1和0.2280 cm3·g−1。为了进一步说明TC分子与两种CTFs孔径之间的相互作用,图5比较了两种CTFs材料吸附TC后的孔径分布变化。从图5a可知,CTF-1吸附TC前后,孔体积几乎没有变化,孔隙总体积从0.4000 cm3 g−1下降至0.3920 cm3·g−1,比表面积从782.4 m2·g−1下降至748.6 m2·g−1,主要是因为TC不能进入CTF-1微孔孔道,从而孔隙总体积和比表面积变化轻微。而从图5b可以明显的看出,CTFDCBP吸附TC后孔体积显著下降,这归咎于TC进入了CTFDCBP中孔孔道内部,占据了其内部空间导致孔体积减小,吸附TC前后,CTFDCBP比表面积从1745 m2·g−1下降至220.5 m2·g−1,孔隙总体积从1.420 cm3·g−1减小至0.2280 cm3·g−1,也进一步表明TC进入到了CTFDCBP孔道内部,CTFDCBP对TC的表现出强烈的吸附选择性。
-
pH对CTFs吸附TC影响如图6所示。由图6可知,当pH<3.3时,吸附Kd值随pH值增加不断升高,当pH>3.3时,吸附Kd值随pH值增加逐渐降低。当pH<3.3时TC以阳离子形式存在[28],CTFs等电点在pH为7.0左右[21],TC和CTF-1之间存在强烈的静电斥力作用,随着pH增加,TC转变为两性离子,静电斥力作用力降低,导致Kd值增加。但随着pH进一步增加,TC带阳离子的氨基官能团和质子化的烯醇官能团发生了去质子化作用,减轻了TC作为π电子受体的能力,减弱了TC与CTF-1结构间产生的阳离子-π 键作用和π-π 电子交互作用故而造成吸附Kd值逐渐下降。当pH>9时,(pKa3为9.69)TC逐渐转变成阴离子形式,由于其与CTF-1之间静电斥力作用导致Kd值持续下降。
TC在CTFDCBP显示了不同的pH影响趋势,Kd值在3—9之间随pH增加不断变大,当pH>9时,静电斥力作用导致Kd值逐渐降低。当pH>3.3时,理论上随着阳离子-π键作用和π-π 电子交互作用减弱会导致Kd值减低,但实际是Kd值却逐步上升,其可能的原因是pH值为3.3—9时,TC主要以中性分子形式存在,由于孔道作用,TC容易进入到CTFDCBP中孔孔道内部,能排除和排斥出空腔中的水分子,从而降低“脱溶剂损失”作用力;同时CTFDCBP的空腔吸附了与之匹配的TC分子,可以使得其在空腔内重新定位,更加容易受到氢键的作用力[18]。
-
离子强度对TC在两种CTFs上吸附的影响如图7所示。对比图7a和b可以发现,离子强度对CTF-1和CTFDCBP吸附TC的影响变化趋势相似,随着离子强度浓度的增加,TC在两种CTFs上吸附的Kd值逐渐降低。在相同离子浓度条件下,氯化钙对CTFs吸附TC的Kd值降低程度要高于以氯化钠。实验pH条件下,TC以两性离子形式存在,CTFs材料质子化而带正电,阳离子(Na+和Ca2+)加入后会产生电子屏蔽作用,降低了两者之间的阳离子-π 键作用。与单价的Na+相比,二价的Ca2+造成电子屏蔽作用更大,因此其对吸附Kd值的影响更大。此外,CTFDCBP对TC的吸附除了阳离子-π 键作用外,还存在着强烈孔道作用。电子屏蔽作用能减弱阳离子-π 键作用,但不会对孔道作用产生影响,导致Kd值在CTF-1下降程度大于CTFDCBP。
-
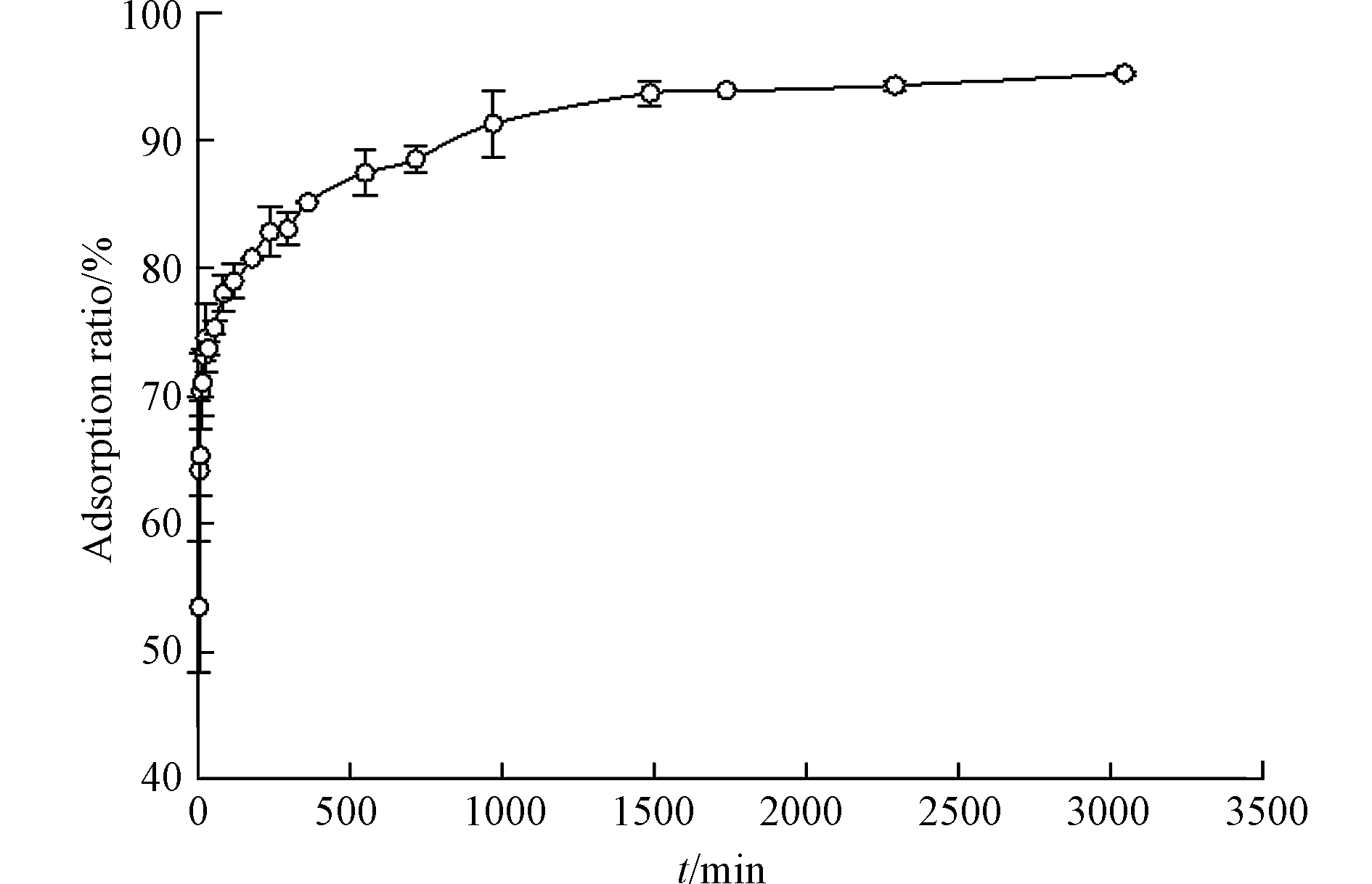
图8显示了TC在CTFDCBP上的吸附动力学。从图8可知,CTFDCBP对TC的吸附在1500 min 内达到平衡,显著低于TC在活性炭和纳米碳管(3d)上吸附动力学平衡时间[29]。主要是因为活性炭是由小的石墨片层组成的网状结构,具有不规则的闭合孔径结构和较大范围的孔径分布。纳米碳管由于疏水作用团聚形成高度不规则的间隙孔径,也具有不均一的孔径分布。与前两者不同,CTFDCBP虽然是无定形结构,但是CTFDCBP具有开放的孔径分布,并且CTFDCBP含有大量的中孔,有利于TC扩散,很快达到吸附平衡。
采用拟一级动力学和拟二级动力学模型对动力学数据进行了拟合,拟合参数见表3。结果表明,拟一级动力学方程拟合的相关系数较低,并且计算的平衡吸附量值与实验值相差甚远。而拟二级动力学拟合的相关系数(R2=0.9990)高,且拟合的平衡吸附量与实验吸附量值非常接近,因此,CTFDCBP吸附TC遵循拟二级动力学方模型,吸附过程中吸附速率受化学吸附影响。
-
制备了两种CTFs吸附剂材料,表征结果表明,CTF-1主要是微孔结构,比表面积为782.4 m2·g−1,CTFDCBP兼具中孔和微孔结构,表面积为1745 m2·g−1。CTFs对TC的吸附等温线符合Freundlich 模型,其吸附吸附作用力包括静电作用力、阳离子-π键作用和π-π电子交互作用。CTFDCBP的中孔结构能有效的避免孔径尺寸排斥效应,对TC具有较好的吸附选择性和快速吸附动力学。制备CTFDCBP在去除抗生素TC方面具有较好的应用前景。
共价三嗪多孔聚合材料对水中四环素的吸附行为及其机理
Adsorption behavior and mechanism of the porous covalent triazine-based framework for tetracycline in water
-
摘要: 采用离子热共聚法制备了两种共价三嗪多孔材料(CTFs),采用BET、XRD、FTIR表征手段研究了CTFs结构和理化性质,并研究了其对四环素(TC)的吸附性能和机理。表征结果表明,CTF-1仅具有微孔结构,CTFDCBP含有中孔结构,CTFDCBP的孔道结构是无定形的,两种CTFs均存在三嗪结构振动峰。吸附实验结果表明,CTFs对TC的吸附过程符合Freundlich等温吸附模型和准二级动力学模型。孔道尺寸排斥效应的存在,使得CTFDCBP对TC的吸附亲和力比CTF-1高1—2个数量级,吸附TC后,CTF-1比表面积和孔隙总体积变化不大,而CTFDCBP比表面积从1745 m2·g−1下降至220.5 m2·g−1,孔隙总体积从1.420 cm3·g−1减小至0.2280 cm3·g−1。中孔孔道作用存在导致TC在两种CTFs表现了不同的pH影响趋势。电子屏蔽作用导致阳离子降低了TC在CTFs上的吸附。CTFs材料对TC附机理包括静电作用力、阳离子-π 键作用、π-π 电子交互作用和孔道作用。研究结果表明,CTFDCBP是一种高效吸附去除水体中TC潜在的吸附剂。Abstract: Two kinds of covalent triazine frameworks (CTFs) with different pore structures were synthesized by ionothermal method. The crystal structure and physicochemical properties of CTFs were studied using BET, XRD, and FTIR. Batch adsorption experiments were carried out to investigate the adsorption behavior and adsorption mechanism of tetracycline (TC). Characterization results illustrated that only microporous structure was contained in CTF-1 and amorphous mesoporous structure was contained in CTFDCBP. In addition, triazine structure vibration peaks have been detected in both two kinds of CTFs. The adsorption process accorded with the Freundlich isothermal adsorption model and pseudo-secondary kinetic model. The adsorption experiment indicated that the adsorption affinity for TC in CTFDCBP is 1—2 orders of magnitude higher than that in CTF-1. The specific surface area and total pore volume of CTF-1 changed slightly after adsorption of tetracycline, while the specific surface area of CTFDCBP decreased vigorously from 1745 m2·g−1 to 220.5 m2·g−1, and total pore volume decreased from 1.420 cm3·g−1 to 0.2280 cm3·g−1. The effects of pH for TC adsorption on two kinds of CTFs were different due to the mesoporous mechanism. Adsorption of TC on CTFs was reduced because of the electronic shielding. Adsorption mechanisms were proposed including electrostatic interactions, cation-π, π-π electron-donor-acceptor interaction, and pore size interaction with the triazine structure of CTFs. Finding in this study demonstrated that the mesoporous CTFDCBP exhibited great potential as an effective adsorbent for TC removal from water.
-
Key words:
- covalent triazine-based framework /
- tetracycline /
- adsorption mechanism
-

-
表 1 CTF-1和CTFDCBP的比表面积和孔体积参数
Table 1. Specific surface area, and pore volume parameters for CTF-1and CTFDCBP
吸附剂Adsorbent 比表面积/(m2·g−1)
Surface areaaVmicb/(cm3·g−1) Vmesc/(cm3·g−1) Vtd/(cm3·g−1) CTF-1 782.4 0.3500 0.0500 0.4000 CTFDCBP 1745 0.7000 0.7200 1.420 a Brunauer-Emmett-Teller (BET) 方法计算得到;b 微孔体积,由Dubinin-Astakhov 方法计算得到;c 中孔体积,由Vt-Vmic 得到;d 总孔体积,由P/P0 = 0.976 得到.
a Determined by N2 adsorption using the Brunauer–Emmett–Teller (BET) method; b Micropore volume, calculated using the Dubinin–Astakhov method; c Mesopore volume, calculated by Vt-Vmic; d Total pore volume, determined at P/P 0 = 0.976.表 2 TC在CTF-1和CTFDCBP吸附的Freundlich 模型拟合参数
Table 2. Freundlich model fitting parameters for tetracycline (TC) adsorption on CTF-1 and CTFDCBP
TC/Adsorbent KF/(mmol1-n ·Ln·kg-1) n R2 Kd/(L·kg-1) TC/CTF-1 80.00±4.00 0.1400±0.0100 0.9660 103-105 TC/CTFDCBP 2300±90 0.1600±0.0100 0.9800 104-106 表 3 拟一级动力学和拟二级动力学拟合参数
Table 3. Fitting parameters for adsorption kinetics by pseudo-first order and pseudo-second order models.
C0/
(mmol·L−1)qexpa/
(mmol·kg−1)拟一级动力学方程b 拟二级动力学方程c k1/
(min−1)qcald/
(mmol·kg−1)R2 k2/
(kg· mmol−1·min−1)qcald/
(mmol·kg−1)R2 0.2250 1068 2.710×10−4 250.9 0.9110 4.730×10−5 1063 0.9990 a qexp 为平衡吸附量的实验值。b lg(qe-qt) = lg(qe)-k1t/2.303。qe平衡吸附量的拟合值,qt是t时吸附量,k1为拟一级动力学速率常数。c t/qt = 1/(k2qe2)+t/qe。qe平衡吸附量的拟合值,qt是t时吸附量,k2为拟二级动力学速率常数。d 从吸附动力学模型计算得到吸附量.
a qexp is the experimental equilibrium adsorption capacity。b lg(qe-qt) = lg(qe)-k1t/2.303. qe is the equilibrium adsorbed concentration, qt is the adsorbed concentration at time t, k1 is the pseudo-first order rate constant.。c t/qt = 1/(k2qe2)+t/qe. qe is the equilibrium adsorbed concentration, qt is the adsorbed concentration at time t, k1 is the pseudo- second order rate constant.。d The adsorption capacity is calculated from the adsorption kinetic model. -
[1] PAULA FAGUNDES A, FELIPE VIANA da SILVA A, BUENO de MORAIS B, et al. A novel application of bentonite modified with copper ions in the tetracycline adsorption: An experimental design study [J]. Materials Letters, 2021, 291: 129552. doi: 10.1016/j.matlet.2021.129552 [2] LIU H D, XU G R, LI G B. Preparation of porous biochar based on pharmaceutical sludge activated by NaOH and its application in the adsorption of tetracycline [J]. Journal of Colloid and Interface Science, 2021, 587: 271-278. doi: 10.1016/j.jcis.2020.12.014 [3] SHI Z, ZHANG Y, SHEN X F, et al. Fabrication of g-C3N4/BiOBr heterojunctions on carbon fibers as weaveable photocatalyst for degrading tetracycline hydrochloride under visible light [J]. Chemical Engineering Journal, 2020, 386: 124010. doi: 10.1016/j.cej.2020.124010 [4] DEBNATH B, MAJUMDAR M, BHOWMIK M, et al. The effective adsorption of tetracycline onto zirconia nanoparticles synthesized by novel microbial green technology [J]. Journal of Environmental Management, 2020, 261: 110235. doi: 10.1016/j.jenvman.2020.110235 [5] 韩爽, 肖鹏飞. 过硫酸盐活化技术在四环素类抗生素降解中的应用进展 [J]. 环境化学, 2021, 40(9): 2873-2883. doi: 10.7524/j.issn.0254-6108.2020052401 HAN S, XIAO P F. Application progress of persulfate activation technology in degradation of tetracycline antibiotics [J]. Environmental Chemistry, 2021, 40(9): 2873-2883(in Chinese). doi: 10.7524/j.issn.0254-6108.2020052401
[6] QIAO D S, LI Z H, DUAN J Y, et al. Adsorption and photocatalytic degradation mechanism of magnetic graphene oxide/ZnO nanocomposites for tetracycline contaminants [J]. Chemical Engineering Journal, 2020, 400: 125952. doi: 10.1016/j.cej.2020.125952 [7] XIA J, GAO Y X, YU G. Tetracycline removal from aqueous solution using zirconium-based metal-organic frameworks (Zr-MOFs) with different pore size and topology: Adsorption isotherm, kinetic and mechanism studies [J]. Journal of Colloid and Interface Science, 2021, 590: 495-505. doi: 10.1016/j.jcis.2021.01.046 [8] CHEN Y J, LI J N, WANG F H, et al. Adsorption of tetracyclines onto polyethylene microplastics: A combined study of experiment and molecular dynamics simulation [J]. Chemosphere, 2021, 265: 129133. doi: 10.1016/j.chemosphere.2020.129133 [9] LIU G M, ZHENG S R, YIN D Q, et al. Adsorption of aqueous alkylphenol ethoxylate surfactants by mesoporous carbon CMK-3 [J]. Journal of Colloid and Interface Science, 2006, 302(1): 47-53. doi: 10.1016/j.jcis.2006.06.006 [10] KILDUFF J E, KARANFIL T, CHIN Y P, et al. Adsorption of natural organic polyelectrolytes by activated carbon: A size-exclusion chromatography study [J]. Environmental Science & Technology, 1996, 30(4): 1336-1343. [11] YANG J, DOU Y P, YANG H M, et al. A novel porous carbon derived from CO2 for high-efficient tetracycline adsorption: Behavior and mechanism [J]. Applied Surface Science, 2021, 538: 148110. doi: 10.1016/j.apsusc.2020.148110 [12] KUHN P, ANTONIETTI M, THOMAS A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis [J]. Angewandte Chemie International Edition, 2008, 47(18): 3450-3453. doi: 10.1002/anie.200705710 [13] KUHN P, KRÜGER K, THOMAS A, et al. “Everything is surface”: Tunable polymer organic frameworks with ultrahigh dye sorption capacity [J]. Chemical Communications, 2008(44): 5815. doi: 10.1039/b814254h [14] KUHN P, THOMAS A, ANTONIETTI M. Toward tailorable porous organic polymer networks: A high-temperature dynamic polymerization scheme based on aromatic nitriles [J]. Macromolecules, 2009, 42(1): 319-326. doi: 10.1021/ma802322j [15] CHAN-THAW C E, VILLA A, KATEKOMOL P, et al. Covalent triazine framework as catalytic support for liquid phase reaction [J]. Nano Letters, 2010, 10(2): 537-541. doi: 10.1021/nl904082k [16] LI Y J, ZHENG S H, LIU X, et al. Conductive microporous covalent triazine-based framework for high-performance electrochemical capacitive energy storage [J]. Angewandte Chemie (International Ed. in English), 2018, 57(27): 7992-7996. doi: 10.1002/anie.201711169 [17] JENA H, KRISHNARAJ C, WANG G B, et al. Acetylacetone covalent triazine framework: An efficient carbon capture and storage material and a highly stable heterogeneous catalyst [J]. Chemistry of Materials, 2018, 30(12): 4102-4111. doi: 10.1021/acs.chemmater.8b01409 [18] LIU J L, ZONG E M, FU H Y, et al. Adsorption of aromatic compounds on porous covalent triazine-based framework [J]. Journal of Colloid and Interface Science, 2012, 372(1): 99-107. doi: 10.1016/j.jcis.2012.01.011 [19] LIU J L, CHEN H, ZHENG S R, et al. Adsorption of 4, 4'-(propane-2, 2-diyl)diphenol from aqueous solution by a covalent triazine-based framework [J]. Journal of Chemical & Engineering Data, 2013, 58(12): 3557-3562. [20] LIU J L, CAO J J, CHEN H, et al. Adsorptive removal of humic acid from aqueous solution by micro- and mesoporous covalent triazine-based framework [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2015, 481: 276-282. [21] LIU J L, ZHOU D M, XU Z Y, et al. Adsorptive removal of pharmaceutical antibiotics from aqueous solution by porous covalent triazine frameworks [J]. Environmental Pollution, 2017, 226: 379-384. doi: 10.1016/j.envpol.2017.03.063 [22] ZHANG N, ISHAG A, LI Y, et al. Recent investigations and progress in environmental remediation by using covalent organic framework-based adsorption method: A review [J]. Journal of Cleaner Production, 2020, 277: 123360. doi: 10.1016/j.jclepro.2020.123360 [23] ZHANG W, LIANG F, LI C, et al. Microwave-enhanced synthesis of magnetic porous covalent triazine-based framework composites for fast separation of organic dye from aqueous solution [J]. Journal of Hazardous Materials, 2011, 186(2/3): 984-990. [24] AN F X, LIU J L, XU Z Y, et al. Efficient removal of three dyes using porous covalent triazine frameworks: Adsorption mechanism and role of pore distribution [J]. Water Science and Technology, 2020, 82(12): 3023-3031. doi: 10.2166/wst.2020.550 [25] BOJDYS M J, JEROMENOK J, THOMAS A, et al. Rational extension of the family of layered, covalent, triazine-based frameworks with regular porosity [J]. Advanced Materials (Deerfield Beach, Fla. ), 2010, 22(19): 2202-2205. doi: 10.1002/adma.200903436 [26] JI L L, CHEN W, DUAN L, et al. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents [J]. Environmental Science & Technology, 2009, 43(7): 2322-2327. [27] JI L L, CHEN W, BI J, et al. Adsorption of tetracycline on single-walled and multi-walled carbon nanotubes as affected by aqueous solution chemistry [J]. Environmental Toxicology and Chemistry, 2010, 29(12): 2713-2719. doi: 10.1002/etc.350 [28] TOLLS J. Sorption of veterinary pharmaceuticals in soils: A review [J]. Environmental Science & Technology, 2001, 35(17): 3397-3406. [29] JI L L, LIU F L, XU Z Y, et al. Adsorption of pharmaceutical antibiotics on template-synthesized ordered micro- and mesoporous carbons [J]. Environmental Science & Technology, 2010, 44(8): 3116-3122. -



 下载:
下载: