-
近年来,表面处理行业中磷/膦酸盐为主络合剂的镀刷工艺迅速推广,在镀件清洗、镀液更替等过程中产生大量重金属-磷络合废水[1]。由于重金属与磷配体的共存性、交互性和高浓度特质,传统沉淀法和离子交换法均受到的极大冲击,处理效率显著下降,很难实现当前严格的重金属排放标准[2]。此外,重金属的存在也会进一步影响后续对磷的生化处理。目前络合型重金属废水的强化处理技术主要包括置换、光/电/化学氧化破络、电去离子和膜分离等方法,普遍存在成本高、二次污染重、重金属资源回收难等不足[3-5]。如何绿色高效地实现此类废水中重金属的深度处理与资源回收,至今仍是环境领域的热点话题之一。
螯合吸附法因其吸附剂功能基可与重金属离子发生配位等强亲和力,且可脱附再生,因此在复杂废水中重金属资源化去除方面极具潜力[6-7]。此类功能基主要可分为胺基乙酸、胺基磷酸、胺肟、二硫代氨基甲酸及多胺型等。本课题组前期的初探发现多胺型树脂对有机酸、焦磷酸等强络合体系中重金属离子的吸附选择性显著优于其他类型的吸附树脂[8-9]。和目前开展的螯合树脂相比,壳聚糖基微球制备简单、环境友好,不仅自身富含丰富的胺基,而且易于嫁接衍生,成为重金属吸附剂优选基体材料。
本文以焦磷酸镀镍清洗废水中镍的高效去除为目标,制备系列聚胺型壳聚糖微球(ACSx),系统探究其对焦磷酸(pyrophosphate缩写为PP)共存络合体系中镍的吸附特性与机制,并进行动态处理模拟实验,评估应用可行性,以期为研发适于络合型重金属废水深度净化的绿色材料提供理论指导。
-
试剂:无水乙醇、盐酸、硝酸、硫酸、磷酸二氢钾购自南京化学试剂股份有限公司,壳聚糖、环氧氯丙烷、乙酸、氢氧化钠、无水硫酸钠、无水碳酸钾、氯化铵、氯化镍、过硫酸钾、抗坏血酸、钼酸铵、半水酒石酸锑钾购自上海国药集团化学试剂有限公司,草酸钠购自上海凌峰化学试剂有限公司,焦磷酸钾购自罗恩试剂,聚乙烯亚胺(PEI,CAS:9002-98-6,分子量70000,50%水溶液)、腐殖酸(HA)购自上海阿拉丁生化科技股份有限公司。所有试剂均直接使用,未经二次纯化。
仪器:电子天平(ME104E,梅特勒-托利多仪器上海有限公司)、电热恒温鼓风干燥箱(XMTD-8222,上海精宏实验设备有限公司)、pH计(PB-10,赛多利斯上海贸易公司)、紫外/可见光分光光计(P5/P6,上海美谱达仪器有限公司)、恒温振荡培养箱(国华企业)、AAS原子吸收光谱仪(TAS-990,北京普析通用)、立式压力蒸汽灭菌器(BXM-30R,上海博讯医疗生物仪器有限公司)、真空冷冻干燥机(Biosafer-10A,赛飞中国有限公司)、电子能谱仪(XPS,PHI 5000 VersaProbe,日本UlVAC-PHI公司)。
-
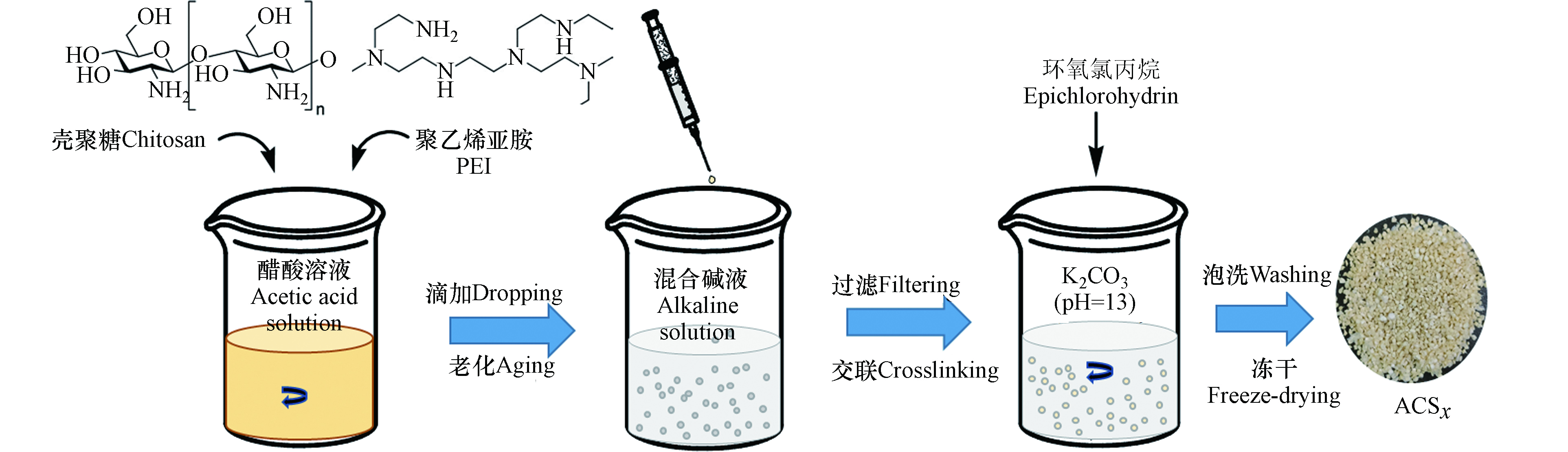
系列聚胺壳聚糖微球的制备方法是参考前期本课题组的报道[10]。如图1所示,将3 g壳聚糖粉末溶解于100 mL体积分数为2%的醋酸溶液搅拌形成淡黄色透明母液。取30 mL母液加入定量体积的PEI溶液在50 ℃搅拌均匀,采用针筒吸取后滴入提前配制的NaOH-Na2CO3混合碱液成球,过滤后将第一级微球加入到含有0.5 mL环氧氯丙烷的饱和碳酸钾溶液(pH=13)中进行后交联稳固,最终过滤、水洗至中性冻干备用。根据PEI的体积为0—1.75 mL,制得系列聚胺壳聚糖微球依次命名为ACS0、ACS0.5、ACS1.0、ACS1.25、ACS1.5、ACS1.75。经光电子能谱扫描测得其中ACS1.5的氮含量为9.7%,通过Zeta电位测定其表面等电点约为pH 9.4。选取国产阳离子交换树脂D001(苯乙烯-二乙烯基苯骨架,磺酸基,硫含量14%,厂家为中国争光)和胺基乙酸型螯合树脂D463((苯乙烯-二乙烯基苯骨架,亚胺基二乙酸基,氮和氧含量分别为4.4%和21.05%,厂家为东大化工)及南京大学研发的多胺型螯合树脂PAMD(苯乙烯-丙烯酸甲酯骨架,多胺基,氮含量为18.6%,授权专利号ZL201310108031.4)为参照吸附剂进行对比。所有吸附树脂在使用前均采用乙醇为洗提剂在索氏提取器中95 ℃抽提12 h,再用纯水洗至中性、冻干备用。
-
配制高浓度的氯化镍和焦磷酸钾储备液,对储备液进行稀释获得预设浓度的镍离子、焦磷酸根及其混合溶液,采用稀盐酸和氢氧化钠调节溶液初始pH。准确称量15 mg各类吸附剂于盛有30 mL上述吸附溶液的玻璃吸附瓶中,确认吸附剂与溶液能够充分接触,振荡反应48 h,转速为160 r·min−1,温度为298 K。吸附剂对比实验中设置初始浓度为镍0.5 mmol·L−1、焦磷酸1 mmol·L−1或4 mmol·L−1,pH值为6和9。根据实际废水水质,加入过量焦磷酸根(2—10 mmol·L−1)或固定焦磷酸浓度为1 mmol·L−1,加入氯化铵(10 mmol·L−1)、硫酸钠(10 mmol·L−1)、腐殖酸(HA,100 mg·L−1)和草酸钠(2 mmol·L−1)作为第三溶质到相应的混合溶液中(初始pH 9),采用相似的操作方法,评估吸附剂对镍的吸附抗干扰性能。等温线实验中镍初始浓度为0.25—3 mmol·L−1,且与焦磷酸初始浓度摩尔比保持1:2,初始pH为9。动力学实验提高吸附液体积至300 mL,保持吸附剂固液比不变,镍初始浓度为0.5 mmol·L−1且焦磷酸为1 mmol·L−1(命此情况为体系1)或4 mmol·L−1(命此情况为体系2),初始pH值为9,间隔一定时间停下立即用移液枪取样(1.5 mL)后继续振荡至平衡。
-
以固定床吸附柱方式评估聚胺壳聚糖微球对焦磷酸-镍络合废水动态处理能力。搭建吸附剂床层体积(BV)为7.5 mL(相应干态吸附剂量为0.6 g),吸附柱为有水套层的玻璃柱(Φ10 mm×240 mm),保持水套层水温为298 K。使用蠕动泵以4 BV·h−1的流量将实验室配制的镍-焦磷酸混合溶液(初始摩尔浓度分别为1 mmol·L−1和2 mmol·L−1)从上方泵入柱中通过微球床的吸附处理,收集出水,每隔特定的时间取样并进行水质分析。吸附饱和后,采用稀盐酸(pH=1.5)以1 BV·h−1泵入床层进行脱附,获得浓缩的镍回收液。
-
水溶液中镍离子(总量)浓度采用原子吸收分光光度计测定,溶液中的PP(总量)采用钼酸铵分光光度计法测定总磷浓度来计算。通过质量平衡原理计算吸附量Q(mmol·g−1)。利用Langmuir和Freundlich等温吸附模型拟合镍的吸附等温线,采用常见的准一级和准二级动力学方程模拟吸附动力学过程[11]。采用Visual MINTEQ软件计算溶液化合物形态,测定吸附前后微球表面元素XPS能谱变化。
-
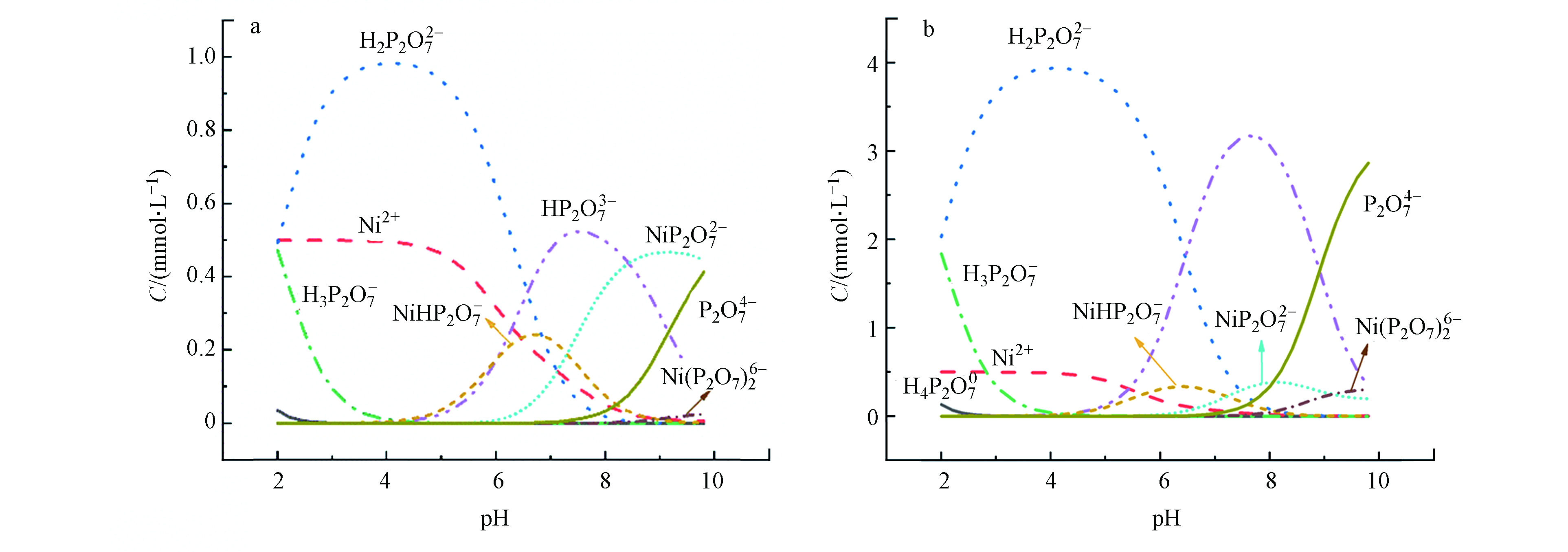
电镀过程中常加入焦磷酸盐与待镀金属在弱碱性条件下发生络合,减缓电沉积过程,满足产品精细要求。因此,清洗废水中含有焦磷酸、镍及其络合物。根据焦磷酸多级解离常数和焦磷酸-镍络合稳定常数[8,12],采用Visual MINTEQ软件,可得出pH2—10条件下,实验设置高、低两种初始浓度比值下各形态组分的浓度值,如图2所示。随着pH的升高焦磷酸与镍离子形成的络合物占比增大,相应游离态镍离子浓度显著降低,NiHP2O7−、NiP2O72−、Ni(P2O7)26−的浓度依次先上升为主形态,之后下降。常见的电镀废水(pH 9)条件下, NiP2O72−、Ni(P2O7)26−为主要的镍赋存形态。焦磷酸根浓度提升(Ni∶PP摩尔比从1∶2提高到1∶8)时,Ni(P2O7)26−增加,且共存大量的非络合态焦磷酸根阴离子。如1:8时络合态和非络合态焦磷酸组分的浓度比值约为1∶3。
-
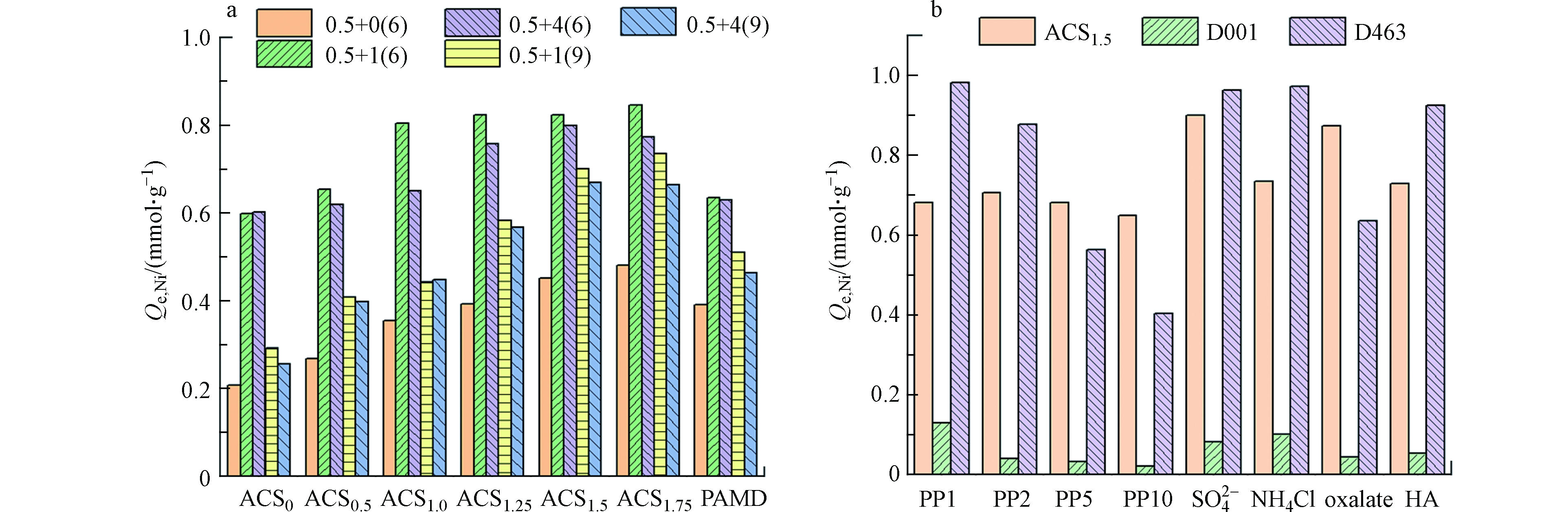
如图3a所示,提高PEI的掺入量,得到系列聚胺壳聚糖微球,其对单组分镍的吸附量逐渐提升,在加入量为1.25 mL时已超越前期制备的多胺树脂PAMD。对于多胺型吸附剂,焦磷酸的存在均能够显著促进镍的吸附,这主要是镍的吸附机制除了游离态Ni2+与胺基的配位作用以外,还增加了[Ni-PP]络合物与质子化胺基的静电作用,因而胺基的利用率更高,镍的吸附量增大[13-14]。pH 9和pH 6相比,相同焦磷酸体系,镍吸附量降低,这是由于胺基去质子化,与[Ni-PP]静电作用削弱。焦磷酸络合体系中镍的吸附量也与PEI加入量基本呈正相关,在投加量大于1.0 mL之后变化较小,可能是由于空间位阻限制了焦磷酸盐的吸附量。相同焦磷酸络合体系,ACS1.0-ACS1.75对镍的吸附量超越PAMD树脂3.2%—43.3%。考虑PEI投加量为1.75 mL时前驱体溶液黏度过大,形成的吸附剂颗粒不规则,优选ACS1.5作为最佳吸附剂开展后续的实验。
实际废水中常共存高浓度的无机盐或有机物,因此镍吸附去除的稳定性和选择性至关重要。如图3b所示,与阳离子交换树脂D001和胺基乙酸型螯合树脂D463相比,ACS1.5对镍的吸附更加稳定,随着焦磷酸浓度从1 mmol·L−1提升至10 mmol·L−1,D001和D463对镍的吸附量分别下降83.2%和58.8%,这是由于碱性条件下镍主要以阴离子型[Ni-PP]络合物存在,其与磺酸基团和胺基乙酸基团相互排斥。而ACS1.5对镍的吸附最大下降5.8%,这是过量的游离态焦磷酸根与阴离子络合物之间发生位点竞争作用导致的,然而,抑制率较低也说明了ACS1.5对后者的亲和选择性更高。另一方面,相同的镍-焦磷酸体系,共存硫酸盐、氯化铵(电镀液缓冲剂)、草酸(电镀辅配位剂)和HA(溶解性有机物模型物),ACS1.5也较D001和D463表现出明显的优势,其对镍的吸附量未降低反而增大了。推测是高浓度的硫酸根和氯离子对质子化胺基有静电屏蔽作用,使得更多的胺基可与镍离子发生配位[15];而液相中草酸与焦磷酸相似也会与镍发生较强的络合作用,因此更多的镍以阴离子络合物的形态吸附于质子化胺基位点上。HA对镍的络合能力相对较弱,其共存时对镍去除影响较小[16]。
-
(1)吸附等温线
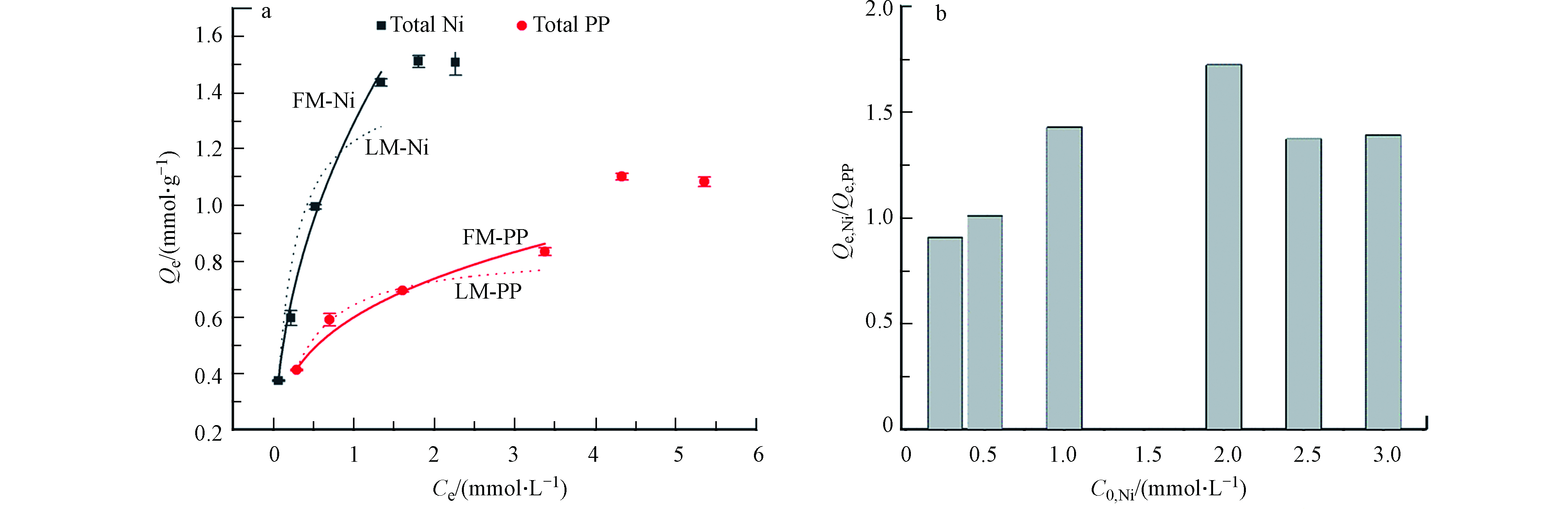
保持Ni(Ⅱ)和PP初始浓度摩尔比1∶2,提升络合体系溶质浓度,获得污染物平衡浓度与ACS1.5吸附量的关系图(即吸附等温线)如图4a所示。
可以发现如预期一致,ACS1.5对Ni(Ⅱ)和PP为同步吸附。Ni(Ⅱ)的吸附随浓度的提升快速上升至平稳,最大实验吸附量为1.54 mmol·g−1;而PP的吸附随浓度上升先缓缓上升,在高浓度时又出现突增,可看作两个阶段变化。采用典型的等温线模型对Ni(Ⅱ)和PP吸附曲线进行拟合发现,中低浓度范围内可高度符合Freundlich模型曲线,其拟合系数大于0.99(表1),这意味着存在一定的多层吸附现象,即Ni(Ⅱ)与PP互相桥连。此外,将Ni(Ⅱ)和PP在ACS1.5上的吸附量比值与初始浓度建立关系发现,实验浓度范围内QNi: QPP从1.0先增大至1.8后又降低至1.5(图4b)。推测原因:一方面,随着镍及其庞大络合物在ACS1.5界面上的累积,存在极大的空间位阻,一定程度导致[Ni-PP]络合物解络[17],PP在固相中负载减少,因而固相中Ni:PP比值超过其液相络合比;另一方面,固相中镍累积到一定程度形成类似“Ni”位点进一步对液相中高浓度游离态PP产生架桥作用[18],从而导致PP吸附量有突增现象直至饱和.
(2)吸附动力学
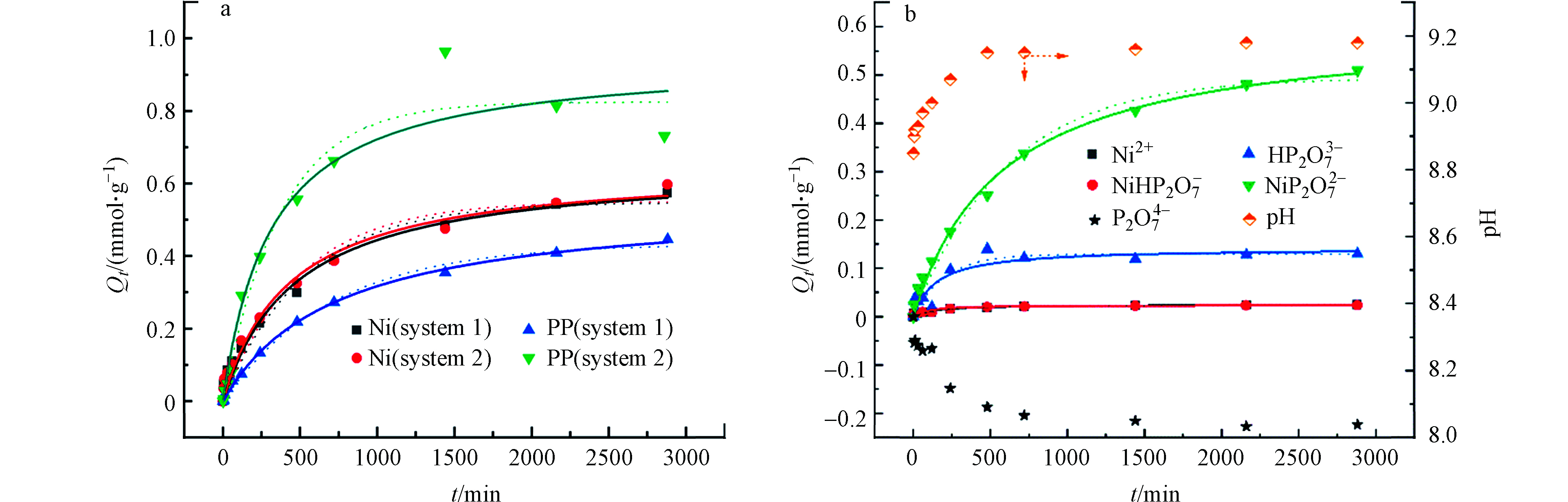
动力学可以进一步观察到污染物与吸附剂的交互过程。镍-焦磷酸摩尔比为1∶2和1∶8的复合体系中ACS1.5对Ni(Ⅱ)和PP的吸附量随时间变化如图5a所示。
采用准一级和准二级动力学方程进行拟合,Ni(Ⅱ)和PP吸附均更符合准二级动力学方程(拟合参数见表2),但例外的是1∶8体系中PP的瞬时浓度在1500 min之后出现回升,即表现出部分PP从固相中释放,从而PP吸附量降低,呈现两个阶段,动力学方程拟合系数均较低。这一现象与等温线实验结果相呼应,进一步证实了随着镍及其络合物在ACS1.5上富集,会导致空间位阻,与ACS1.5或ACS1.5-Ni结合的PP发生了部分解析。这也间接证明了ACS1.5对镍及镍络合物较非金属溶质的吸附亲和力更强、选择性更高。进一步对1∶2体系中各主形态的表观吸附进行分析,如图5b所示,实验条件下该体系主形态有5种,除P2O74−外,其余形态的表观吸附量均随吸附时间的推移,逐渐增大至平衡。游离P2O74−表观吸附为负值,说明其在位点竞争中为弱势组分。随着镍及其复合物在固相的累积,位阻效应导致部分络合的焦磷酸根被解络释放到液相中,因而增加了游离态P2O74−的浓度。对各吸附曲线进行拟合,其中拟二级方程均较拟一级方程拟合系数更高,说明该体系吸附过程包含化学作用机制。其中NiP2O72−的拟合系数高达0.993,而Ni2+和NiHP2O7−拟合系数也超过0.90。值得注意的是,NiP2O72−初始吸附速率常数最大,表明ACS1.5对NiP2O72−吸附亲和力更高。上述结果与前期的讨论相符,由于PP的络合形成[Ni-PP]阴离子型络合物,通过静电吸引作用可快速的富集到表面胺基位点,再由于其组分中镍可与毗邻中性胺基的配位作用,使得NiP2O72−较游离态PP与ACS1.5结合力更高,可高选择性吸附。此外,吸附过程中pH有升高现象,这与前期研究报道一致[8],可解释为由于[Ni-PP]阴离子与胺基发生强静电作用,促使部分胺基夺取了水中的质子带正电,水相中OH−浓度增大。
(3)XPS表征验证
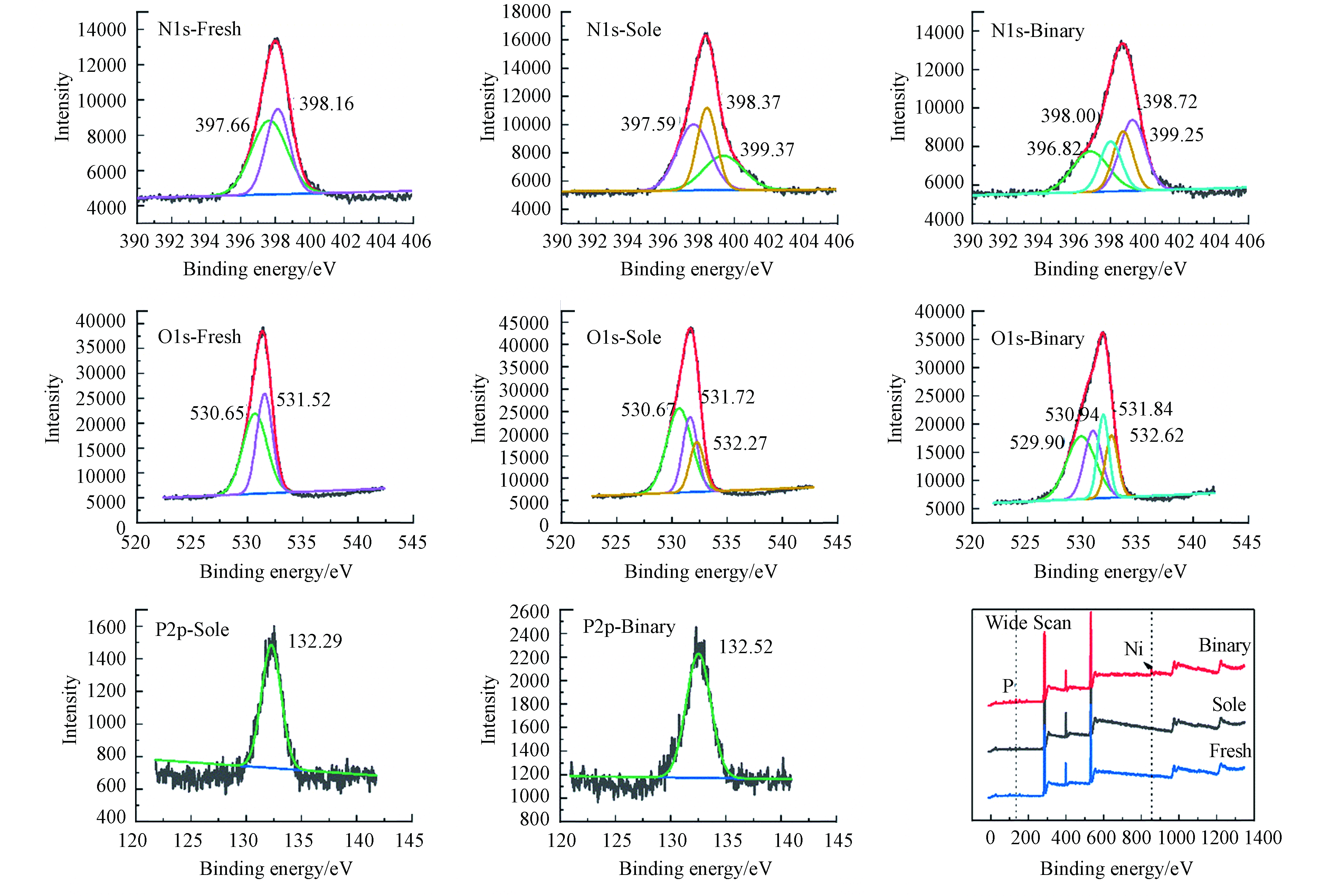
对比不同体系吸附处理后ACS1.5的XPS谱图(图6)可进一步确定参与的吸附位点和微界面交互作用机制。经过单独PP处理和经过Ni-PP处理的ACS1.5与新鲜ACS1.5吸附剂相比,其表面XPS全扫依次增加了磷和镍的谱峰,直观表明焦磷酸与镍在ACS1.5上的富集。
新鲜吸附剂主要由碳氮氧组成,其中N1s的精细谱图中397.66 eV和398.16 eV处峰分别代表中性伯胺基和仲胺基[19],两者质子化带正电时结合能会升高[20]。PP吸附后N增加了399.37 eV处的新峰,这是PP中P—OH与胺基强氢键作用导致,胺基结合水中H+而质子化。当焦磷酸-镍共同吸附后,所有的N1s支峰均发生位移,其中结合能在396.82 eV为胺基与镍发生了配位导致的,而结合能升高的两种N1s支峰(398.72和399.25 eV)同样是PP及[Ni-PP]络合物与固相胺基静电作用,胺基发生了质子化所导致的[8, 19-20]。N1s的变化表明了焦磷酸-镍双组分与ACS1.5表面的胺基同时发生了配位和静电吸附作用。进一步分析O1s的变化:新鲜ACS1.5表面的氧组分由壳聚糖母体自带羟基和偶联剂引入的羟基组成。单独的PP吸附后新增532.27 eV极可能是P=O键的特征峰,而531.5eV向531.72 eV迁移可能是PP负载后多种羟基的叠加。当镍与焦磷酸共吸附后,O1s支峰较单独PP体系中的情况变化极大,其强度在529.9 eV和532.62 eV出现峰值。尽管各种氧环境的结合能位置存在较多的重叠,但这些明显的变化证明了固相中镍与焦磷酸中的含氧基团发生了较强的交互作用(即配位络合)。此外,根据焦磷酸的特征元素磷的结合能(P2p)在镍共吸附时发生了明显的位移(0.23 eV),也可以验证焦磷酸-镍在ACS1.5固相微界面发生了键联作用(-Ni-O-P-)。
结合上述讨论,碱性废水中焦磷酸共存时ACS1.5对镍的吸附主要以NiP2O72−(主成分)形态与聚胺基团发生了配位-静电联合作用,即毗邻质子化胺基和中性胺基分别与NiP2O72−络合物正负两端发生静电和配位作用,结合力加固,镍去除的抗干扰性增强。随着NiP2O72−的富集,焦磷酸根空间位阻增大,导致部分焦磷酸根脱落,而镍仍能够稳定吸附于固相中,因此实现焦磷酸络合废水中镍的高效稳定去除。图7为推测机理的简单示意图。
-
采用固定床动态吸附法评估ACS1.5对焦磷酸-镍络合废水的净化能力(图8)。以出水镍浓度超过0.1 mg·L−1(即GB21900—2008表3限值)为穿透点,流速为4 BV·h−1时,处理18 BV后固定床发生镍穿透。随着处理体积的提升,出水镍浓度逐渐上升,待处理体积为348 BV时,镍出水浓度达进水浓度90%以上,基本饱和,经累积计算镍饱和吸附量可达1.74 mmol·g−1。同时也监控了PP的出水浓度,其达到饱和的时间较镍更快,约为152 BV。采用稀盐酸对饱和后的ACS1.5进行脱附,采用1 BV·h−1的再生流速,在14 BV时镍出水浓度达到最高,为12.37 mmol·L−1,在32 BV后基本脱附完全,脱附率为93.8%,浓缩倍率约为7.67。此外,由于弱酸条件下PP仍能与质子化胺基作用,因此脱附液中PP的占比较废水中明显降低,PP平均浓度为0.099 mmol·L−1。上述结果表明,ACS1.5在实际处理焦磷酸镀镍清洗废水具有较优的潜力。
-
聚胺基的引入不仅可以增加壳聚糖微球对镍的吸附容量,还可以增进焦磷酸络合体系中镍的吸附选择性和稳定性。pH 9时,相同络合体系中ACS1.5对镍的吸附量最大为商用离子交换树脂D001和螯合树脂D463的30和1.6倍。
初始浓度1:2摩尔比下,较低浓度范围内镍和焦磷酸吸附等温线均高度符合Freundlich模型,NiP2O72−表观吸附动力学高度符合拟二级动力学方程,初始吸附速率常数最大,为优势吸附组分。NiP2O72−可与聚胺基团中质子化胺基和中性胺基发生静电-配位耦合作用,随着络合物在固相中的累积,发生空间位阻导致部分焦磷酸根解络释放。
ACS1.5固定床动态处理焦磷酸-镍废水,出水18 BV镍浓度低于0.1 mg·L−1,镍饱和吸附容量为1.74 mmol·g−1,稀盐酸可完全再生获得高浓度镍回收液。ACS1.5及类似聚胺型壳聚糖微球有望在络合型重金属废水深度处理与资源化方面发挥作用。
聚胺壳聚糖微球对焦磷酸络合废水中镍的高效选择性去除特性与机制
Efficient removal of Ni(Ⅱ) from pyrophosphate-plating wastewater using the polyamine-grafted chitosan beads
-
摘要: 针对含磷络合型电镀废水高效处理难题,本文探究了系列聚胺型壳聚糖微球(ACSx)对焦磷酸络合体系中镍离子的选择性去除特性。研究表明,焦磷酸的存在显著促进了ACSx对镍的吸附。相同条件下,ACS1.0—ACS1.75对镍的吸附量超越前期报道的PAMD树脂3.2%—43.3%。过量的焦磷酸或其他常见共存物对ACS1.5吸附镍的抑制率小于6%,其抗干扰能力远优于商品化树脂D001和D467。借助Visual MINTEQ形态计算和吸附等温线、动力学模型拟合以及XPS表征分析发现,络合体系中,NiP2O72-为主要的存在形态和吸附形态,其表观吸附动力学曲线高度符合拟二级动力学方程,初始吸附速率常数高于其他形态。推测NiP2O72−与ACS1.5表面聚胺基团(含质子化胺基和中性胺基)发生了静电-配位耦合作用(-N0-NiP2O72−·N+-),抗干扰性强。随着NiP2O72−的富集,焦磷酸配体空间位阻增大,导致部分焦磷酸根脱落,固相镍-焦磷酸吸附量比大于1。固定床动态吸附实验表明镍0.5 mmol·L−1-焦磷酸1 mmol·L−1初始浓度下,流速4 BV·h−1,前18 BV出水镍浓度低于0.1 mg·L−1,镍饱和吸附容量为1.74 mmol·g−1。采用稀盐酸再生,镍脱附率为93.8%,浓缩倍率约为7.67倍。本研究表明ACS1.5在实际处理焦磷酸镀镍清洗废水中具有较优的潜力。Abstract: To improve the advanced treatment of P-containing complexed plating wastewaters, the selective adsorption properties of Ni(Ⅱ) by series of polyamine-grafted chitosan beads (ACSx) were investigated in various pyrophosphate (PP) complexed systems. The results showed that the presence of PP markedly promoted Ni(Ⅱ) adsorption by ACSx. Under the same condition, Ni(Ⅱ) adsorption by ACS1.0-ACS1.75 was 3.2%—43.3% higher than those by the PAMD resin. The inhibition rate of excessive PP or other common coexistent on the adsorption of Ni(Ⅱ) by ACS1.5 was less than 6%, and its anti-interference ability was much better than that of commercial resins D001 and D467. According to the Visual MINTEQ speciation calculation, adsorption isotherm/kinetic models fitting and XPS characterization, NiP2O72- was the main species and adsorbate in the Ni-PP complexed systems. Its apparent adsorption kinetic curve was well fitted by pseudo-second-order kinetic equation. The fitted initial adsorption rate constant of NiP2O72− was higher than those of other species. It was speculated that NiP2O72− has a coupling effect of coordination and electrostatic interaction (-N0-NiP2O72-·N+-) with the polyamine groups containing both neutral amines and protonated amines, leading to the strong anti-interference ability. Along with the accumulation of NiP2O72− onto ACS1.5, part of PP decomplexed and released into the aqueous phase probably due to the steric hindrance, and the ratio of adsorption amount of Ni(Ⅱ) and PP was higher than 1. Furthermore, fixed-column adsorption tests showed that at C0,Ni=0.5 mmol·L−1, C0,PP=1.0 mmol·L−1 and 4 BV·h−1 of the flow rate, Ni(Ⅱ) concentration was lower than 0.1 mg·L−1 in the first 18 BV effluent and the saturated adsorption amount of Ni(Ⅱ) was 1.74 mmol·g−1. In addition, 93.8% Ni(Ⅱ) could be recovered by diluted HCl, and the concentration ratio of Ni(Ⅱ) was about 7.67. The above trends suggest that the application of ACS1.5 in treating pyrophosphate-involved nickel-plating rinse wastewater is highly promising.
-
Key words:
- polyamine group /
- complexed heavy metal ions /
- anti-interference /
- adsorption mechanism
-
多环芳烃(polycyclic aromatic hydrocarbons,以下简称PAHs)对人体具有强烈的致癌、致畸、致突变效应,且在环境中广泛存在 [1-3]。燃煤电厂是环境中大气污染物的重要来源[4-5]。我国燃煤电厂广泛配备的脱硝、除尘和脱硫设施虽然可以高效去除NOx、SO2、粉尘等常规大气污染物,却难以去除更容易进入人体肺部的可过滤细颗粒物、可凝结颗粒物和气态PAHs[6-8]。
近年来,燃煤电厂大气污染物的排放因子和排放清单成为大气污染防治领域的研究热点,并且在识别燃煤电厂大气污染物排放特征、时空分布、以及区域环境效应等方面取得重要进展[9-13]。燃煤电厂排放PAHs的研究主要关注燃煤产物中PAHs的含量、分布和赋存状态的分析,以及燃煤锅炉烟气排放过程PAHs的赋存规律及影响因素等方面[13-19],而关于燃煤电厂PAHs的排放因子和清单的研究较少,尤其缺乏不同燃煤锅炉大气PAHs的精细化排放因子以及其历史排放通量的基础数据。基于此,本研究以安徽省为例,结合资料收集、实地调研和现场实测数据,“自下而上”构建了安徽燃煤电厂大气PAHs的历史排放清单,以查明锅炉、机组、大气污染控制设施以及超低排放改造对安徽燃煤电厂PAHs历史排放量的贡献值。
1. 材料与方法(Materials and methods)
1.1 锅炉机组和大气污染控制设施地理分布
实地调研了安徽省86家燃煤电厂的锅炉机组、大气污染控制设施、燃煤量、产灰量等详细信息,并结合安徽省环保局内部资料,构建了安徽省燃煤电厂基本信息数据库。该数据囊括2010—2017年安徽省173台在役燃煤机组和21台退役机组。基本信息包括位置、机组容量、锅炉类型、燃料类型(煤、煤矸石、煤泥)、煤炭年消费量、大气污染控制设施(脱硝、脱硫、除尘)和机组年龄。截至2017年底,安徽在役燃煤电厂的锅炉机组年龄跨度为0—32年,平均年龄为11年。这些电厂分布在除黄山以外的15个城市。其中煤粉炉95台,流化床炉75台,链条炉3台。煤粉炉装机容量为43.9 GW,占总装机容量的92.4%。流化床炉装机容量为3.4 GW,占总装机容量的7.6%。
选择性催化还原脱硝(SCR)和非选择性催化还原脱硝(SNCR)是安徽燃煤电厂的主要脱硝技术。煤粉炉均装配了SCR和湿式脱硫设备(WFGDs,石灰石-石膏湿法、氨法、电石渣湿法脱硫)。流化床炉装配SCR占其装机容量的75%,其余均装配了SNCR。炉内喷钙是流化床炉的主要脱硫方式,占其装机容量的86%。其余流化床炉装配了WFGDs(占装机容量的7.8%)和半干法脱硫(占装机容量的6.3%)。此外,所有煤粉炉和流化床炉均装配了除尘装置。
1.2 燃煤电厂关闭和更新锅炉情况
2010年至2017年间,安徽燃煤电厂发电量从142.0 TWh增至231.1 TWh[20]。与此同时,煤炭消费量从61.8 Mt增至87.4 Mt,远小于发电量的增长速率,说明燃煤电厂锅炉机组发电效率的显著提升。在此期间,安徽共新建了48台燃煤锅炉,装机容量共计21.5 GW,占2017年安徽燃煤电厂总装机容量的45%。此外,截止2017年底,装机容量≥300 MW的大锅炉占全省总装机容量的93.7%,而装机容量≤30 MW的小锅炉占全省总装机容量的1.7%。由于“上大压小”政策的实施,安徽燃煤电厂平均能耗从2010年的322 gce·kWh−1降至2017年的301 gce·kWh−1[20]。
1.3 排放清单构建
基于不同的锅炉类型和大气污染控制设施,将安徽省173台发电燃煤锅炉分为6类:煤粉炉1(600 MW≤机组容量≤1000 MW),煤粉炉2(300 MW≤机组容量<600 MW),煤粉炉3(6 MW≤机组容量≤210 MW),流化床炉1(6 MW≤机组容量≤210 MW),流化床炉2(3 MW≤机组容量≤60 MW),流化床炉3(3 MW≤机组容量≤15 MW)。
排放因子(EFPAH)基于实测计算得出:EFPAH = V×CPAH/Ccoal,其中V是烟气流速(m3·h−1),CPAH是烟气中PAHs的浓度(µg·m−1),Ccoal是锅炉每小时煤炭消费量(tons·h−1)。计算得出6种燃煤锅炉PAHs的排放因子(表1)。PAHs排放清单基于每一台锅炉的排放因子和煤炭消耗量计算得出:
表 1 典型燃煤锅炉现场试验的基本信息Table 1. Basic information of the six coal-fired units in field tests锅炉Boiler 城市City 机组容量/MWCapacity 燃煤类型Coal typea APCDb 燃煤效率/(t·h−1)Combustion efficiency 烟气流速/(m·s−1)Velocity of flue gas 样本Samplesc PAHs数量Number of PAHs 煤粉炉1 淮南 600 BC SCR+WFGD+ESP 95.8 9.3 P+G 16 煤粉炉2 淮南 300 BC SCR+WFGD+ESP 37.8 10.4 P+G 16 煤粉炉3 淮南 60 BC SCR+WFGD+ESP 18.7 8.7 P+G 16 流化床炉1 淮南 300 BC, CG或IC SNCR+IFSC+FF 53.6 9.8 P+G 16 流化床炉2 淮北 15 BC, CG或IC SNCR+WFGD+FF 25.2 8.3 P+G 16 流化床炉3 淮北 15 BC, CG或IC SNCR+ISFC+FF 9.1 9.7 P+G 16 注:a, BC:烟煤,CG:煤矸石,IC:低质煤;b, SCR:选择性催化还原脱硝,SNCR:非选择性催化还原脱硝,WFGD:湿式脱硫,IFSC:炉内喷钙脱硫,ESP:静电除尘,FF:袋式除尘;c, P:颗粒相,G:气相. Note: a, BC: bituminous coal, CG: coal gangue, IC: inferior coal; b, SCR: selective catalytic reduction, SNCR: selective non-catalytic reduction, WFGD: wet flue gas desulfurization, IFSC: in-furnace spraying calcium, ESP: electrostatic precipitator, FF: fabric filter; P: particulate, G: gaseous. E = ∑i∑k∑m Mi × EFi,k,m (1) 其中,E是燃煤锅炉年排放量(kg·a−1),M是每一台燃煤锅炉的耗煤量(tons),EF是每一种锅炉类型的PAHs的排放因子,i、k、m分别是锅炉类型、机组容量和大气污染控制设施类型。锅炉煤炭消耗量数据来自安徽省环保局。
1.4 质量控制与保证
PAHs使用内标法定量, 以9个不同浓度梯度的PAHs标准溶液(10、20、50、100、200、500、1000、2000、5000 ng·mL−1)绘制标准曲线。所有化合物的标准曲线的回归系数R2均大于0.99。样品中回收率指示物萘-d10、菲-d10、芘-d12、苊-d10和䓛-d12的回收效率分别为56%±12%、73%±13%、69%±10%、84%±11%和71%±14%,方法检测限为0.009—0.017 ng·g−1,在可接受的范围以内。
1.5 不确定性分析
为了定量结果的不确定性,以及各因子对预测结果的影响,应用蒙特卡罗模型模拟2010年至2017年安徽燃煤电厂PAHs的年排放量,所有参数均假设为对数正态分布。蒙特卡罗模拟在Crystal Ball 7.2中运行10000次。
2. 结果与讨论 (Results and discussion)
2.1 不同类型燃煤锅炉PAHs的排放因子和通量
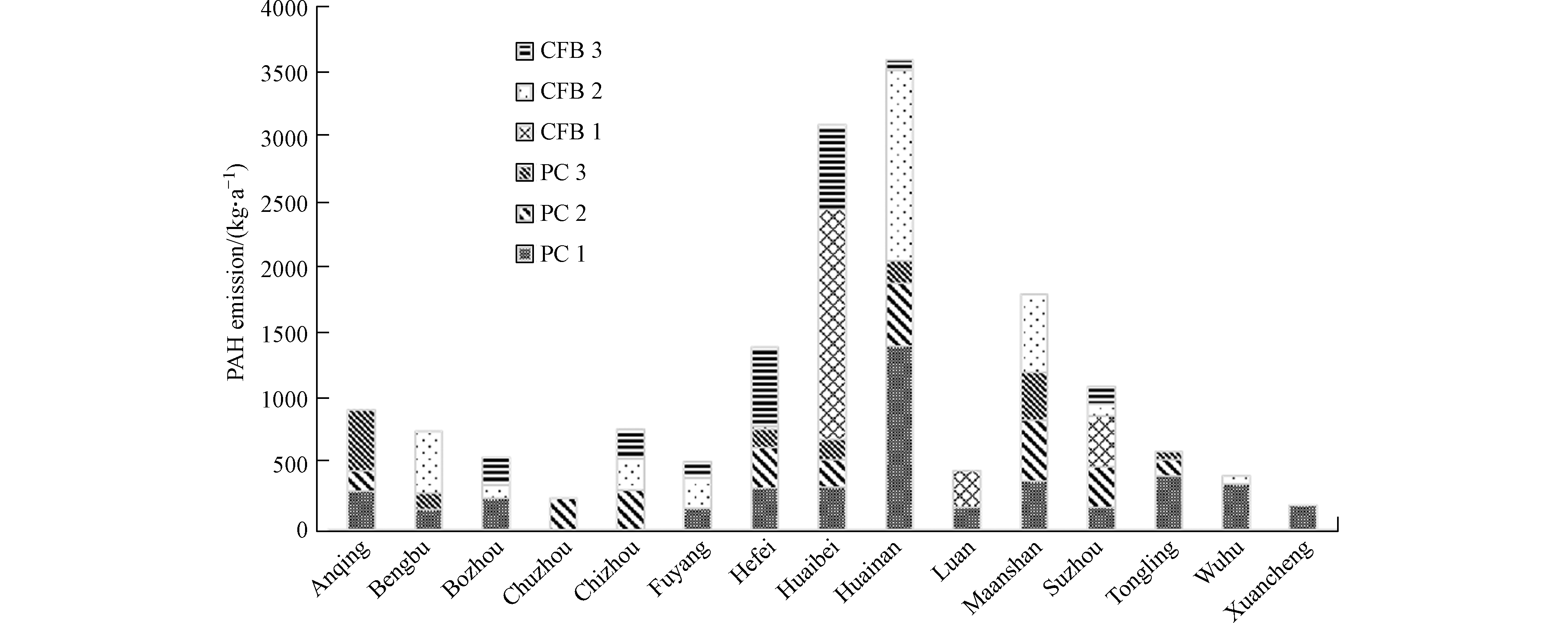
2017年,安徽燃煤电厂PAHs的大气排放量为16400 kg,煤粉炉为8600 kg,流化床炉为7800 kg。其中煤粉炉1、煤粉炉2、煤粉炉3、流化床炉1、流化床炉2、流化床炉3的PAHs排放量分别占比27.9%、15.8%、8.9%、14.9%、20.2%、12.3%(图1)。这6种锅炉的燃煤量占比分别为69.6%、13.8%、4.0%、7.0%、2.8%和2.2%。这表明装机容量更大的锅炉由于燃烧效率更高,且大气污染控制设施条件更先进,从而污染物排放的相对量更小。细分至不同城市级别,淮南(3600 kg)、淮北(3100 kg)和马鞍山(1800 kg)的排放量最大。这主要因为3座城市电厂燃煤量相对较大。此外,安徽南部城市(铜陵、芜湖和宣城)由于燃煤电厂主要为大锅炉(煤粉炉1),导致PAHs相对排放量远小于北部城市(淮北、亳州)和中部城市(合肥)。
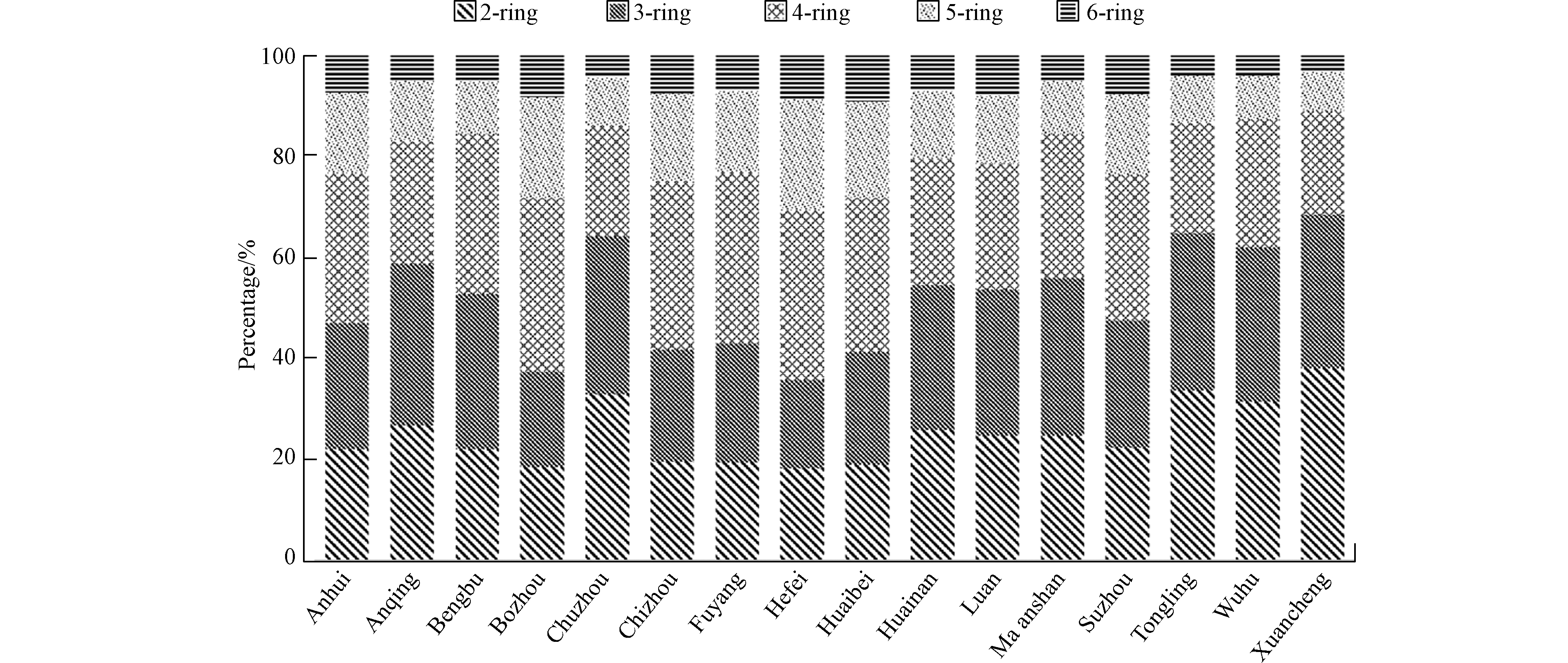
燃煤电厂排放的PAHs以4环PAHs为主,贡献值为28.0%,其次为3环PAHs(占比26.8%)和2环PAHs(占比25.0%)(图2)。中部和北部城市燃煤电厂排放PAHs中的高分子量PAHs(5环和6环PAHs)比南部城市的相对贡献率更高。主要原因是:1)小锅炉(煤粉炉3和流化床炉3)主要分布在中部和北部城市,而它们排放高分子量PAHs的量更大;2)南部城市燃煤电厂的流化床炉装配WFGD的比例更高,而WFGD对于高分子量PAHs的脱除效率更高[6]。
PAHs的排放率(kg·t−1)和排放强度(kg·MW−1),分别定义为PAHs排放量与煤炭消费量,以及PAHs排放量与机组装机容量的比值,表现为煤粉炉 (分别为1.1 kg·t−1和0.19 kg·MW−1)显著大于流化床炉 (7.2 kg·t−1和2.1 kg·MW−1)。这主要是由于流化床炉燃烧温度相对更低(800—1000 ℃),因此比煤粉炉排放PAHs的相对量更大[6]。机组容量相对较大的煤粉炉1、煤粉炉2和流化床炉1比其它锅炉的排放率和排放强度更小(表2),原因是它们具有更高的燃烧温度和锅炉压力。此外,流化床炉3的PAHs排放率和排放强度更高(表2)。
表 2 锅炉机组基本信息和PAHs排放量、排放率、排放强度Table 2. Basic information and PAH emissions, emission rates, and emission intensities from different coal-fired units锅炉Boiler 煤炭消耗量(×104) /t Coal consumptions 总装机容量/MWTotalcapacities 脱氮装置Denitrification device 脱硫装置Desulphurization device 除尘装置De-dust device PAHs排放因子/(μg·kg−1) PAHemission factors PAHs排放量/kgPAH emission amount PAHs排放率/(kg·t−1)PAH emission rates PAHs排放强度/(kg·MW−1)PAH emission intensities 煤粉炉1 6120 35,340 SCR WFGD ESPs 75 4600 0.75 0.13 煤粉炉2 1220 7780 SCR WFGD ESPs 210 2550 2.1 0.32 煤粉炉3 350 1064 SCR WFGD ESPs或FFs 420 1450 4.1 1.4 流化床炉1 620 2808 SCR或SNCR IFSC ESPs或FFs 620 3800 6.1 1.4 流化床炉2 250 487 SCR或SNCR WFGD ESPs或FFs 740 1850 7.4 3.8 流化床炉3 190 300 SCR或SNCR IFSC ESPs或FFs 1150 2150 11.3 7.2 2.2 不同年龄和机组容量PAHs排放通量对比
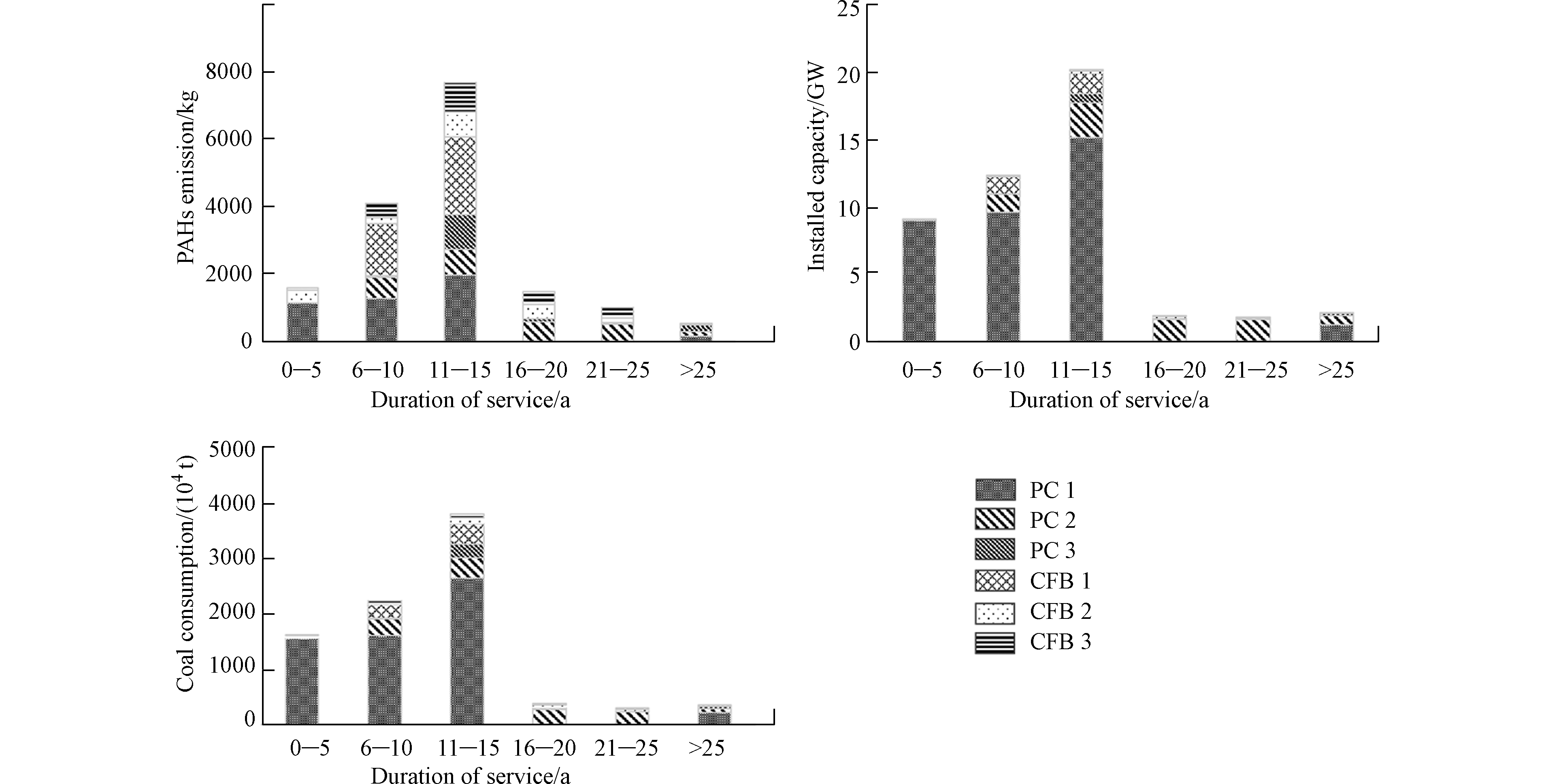
将燃煤锅炉按年龄分为6类:0—5年(2015—2017),6—10年(2010–2014),11—15年(2005—2009),16—20年(2000—2004),21—25年(1995—1999)和> 25年(1986—1994)。从图3可知,11—15年的燃煤锅炉对PAHs排放总量的贡献最大,占46.6%,其次为6—10年和0—5年的锅炉,分别占比25.0%和9.8%(图3)。从不同年龄锅炉的PAHs排放量主要受装机容量和燃煤量的影响:大机组锅炉(煤粉炉1、煤粉炉2和流化床炉1)是较新锅炉(0—5、6—10、11—15年)PAHs排放量的主要贡献者;小机组锅炉(煤粉炉3,流化床炉2和流化床炉3)则是较老锅炉(年龄段为16—20、21—25、>25年)PAHs排放量的主要贡献者(图3)。
2.3 安徽燃煤电厂PAHs的历史排放通量
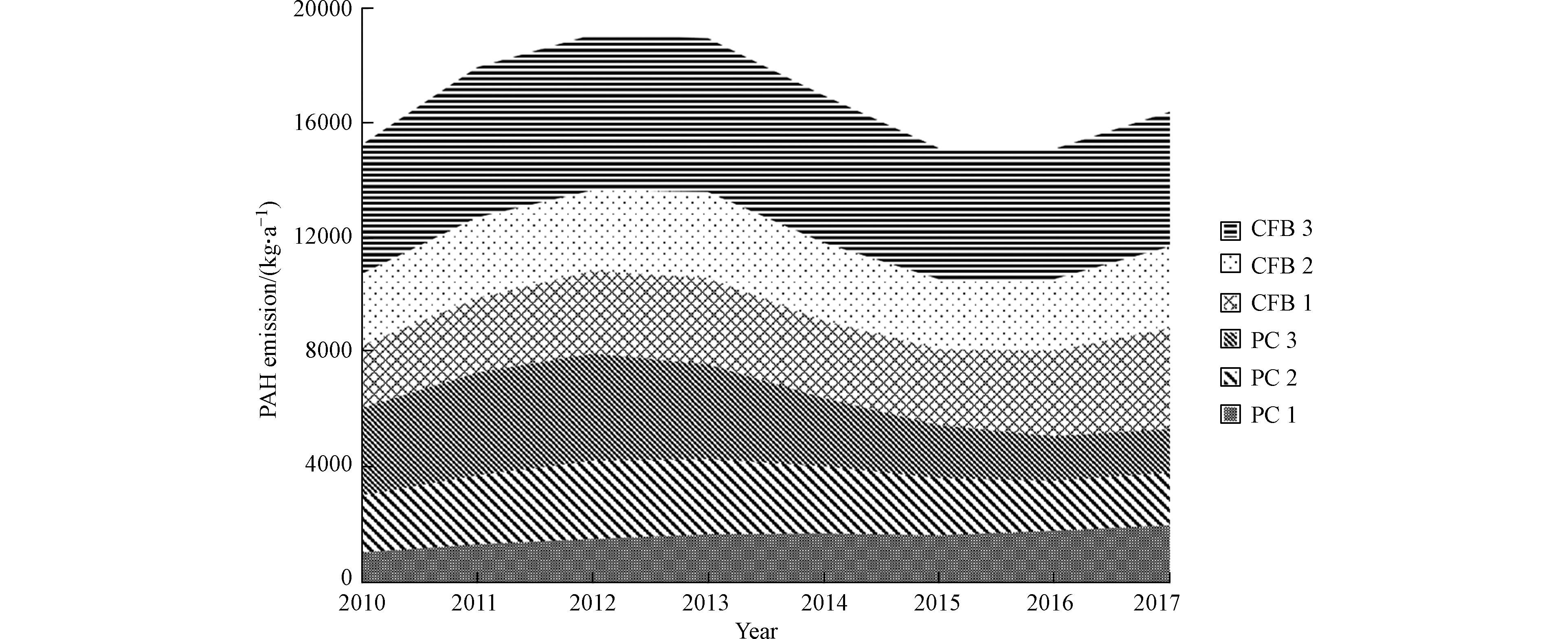
从2010年至2017年,安徽省燃煤电厂大气PAHs的排放量从15200 kg增加至16400 kg,年均增长1.1%。该增长率低于煤炭消费量增长率(5.9%)和发电量的增长率(9.0%)。在6种不同类型的锅炉中,流化床炉3和煤粉炉3的贡献量最大,约分别占总排放量的28.2%—30.2%和14.0%—19.9%。然而,煤粉炉3的贡献量自2013年呈现明显的下降趋势(如图4),这主要是因为安徽省在2013至2017的5年时间里,共取缔了17家小型燃煤电厂,其中取缔的PC3机组占煤粉炉3总机组容量的89.2%—92.7%,导致煤粉炉3锅炉排放PAHs逐年减少。
通过计算得出,自2013年以来,由于关停小电厂导致多环芳烃排放减少的总量为1880 kg。此外,中国于2014年颁布了《全面实施燃煤电厂超低排放和节能改造工作方案》,对燃煤电厂进行全面改造。截止2017年底,安徽省共对67台锅炉机组进行超低排放改造,计算得出截止2017年,经过超低排放改造,安徽省燃煤电厂减少PAHs排放量为760 kg。
2.4 PAHs历史排放量与气温和用电量之间的耦合关系探讨
安徽省燃煤电厂的发电量、煤炭消费量和PAHs的排放量之间具有十分密切的联系(图5)。
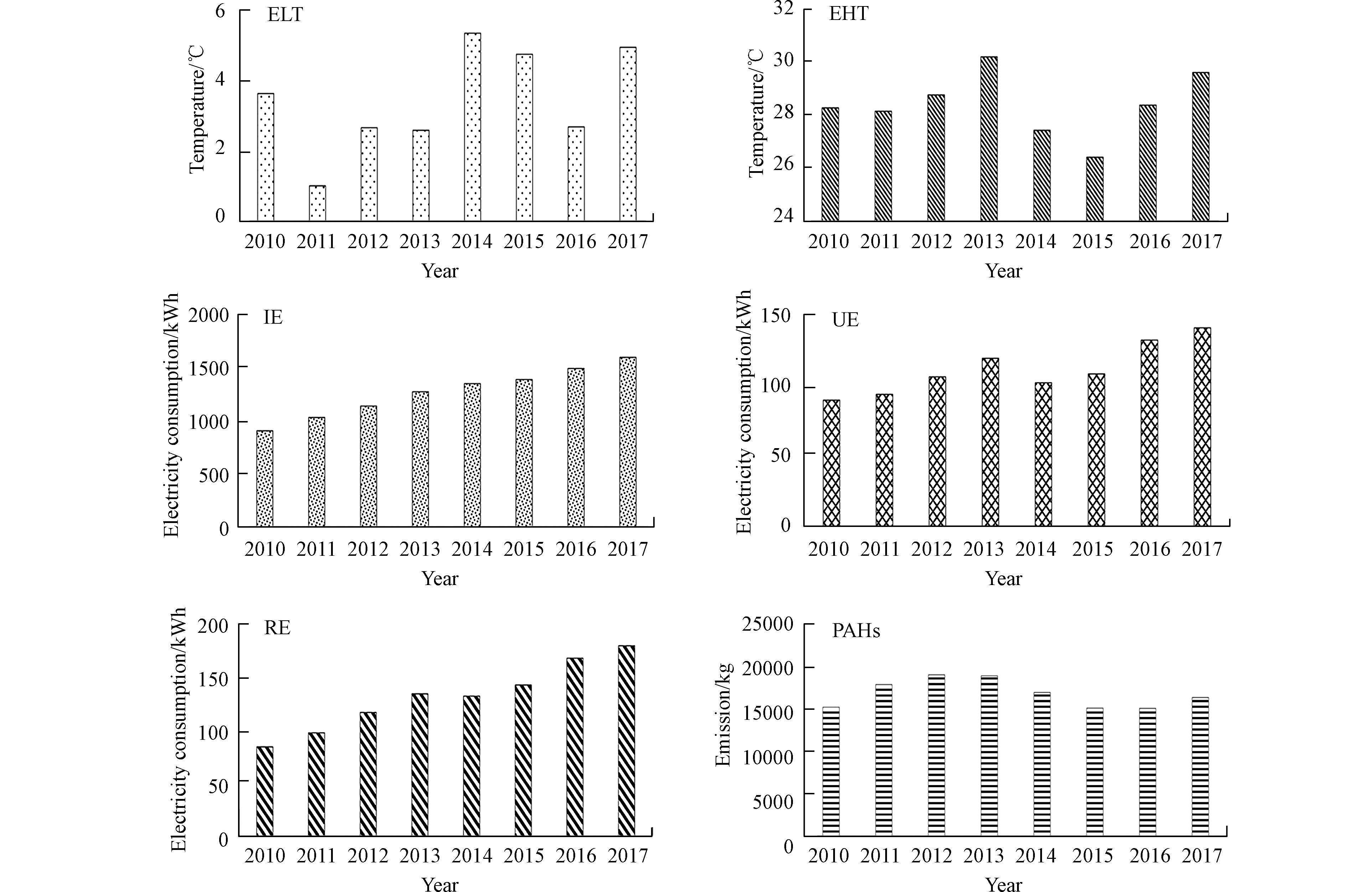 图 5 2010—2017年PAHs年排放量(PAHs)、居民用电量(RE)、城市用电量(UE)、工业用电量(IE)、高温值(EHT)、低温值(ELT)的lg值变化Figure 5. The logarithmic values of PAH emissions (PAHs), resident electricity consumption (RE), urban electricity consumption (UE), industry-wide electricity consumption (IE), extreme high temperature (EHT), and extreme low temperature (ELT) from 2010 to 2017 in Anhui
图 5 2010—2017年PAHs年排放量(PAHs)、居民用电量(RE)、城市用电量(UE)、工业用电量(IE)、高温值(EHT)、低温值(ELT)的lg值变化Figure 5. The logarithmic values of PAH emissions (PAHs), resident electricity consumption (RE), urban electricity consumption (UE), industry-wide electricity consumption (IE), extreme high temperature (EHT), and extreme low temperature (ELT) from 2010 to 2017 in Anhui通过研究发现,2010年至2017年,安徽燃煤电厂PAHs排放量的年度变化与安徽省夏季和冬季平均气温有非常有趣的相关性,例如:2011年安徽省经历了一个“冷冬”(1997年以来最低),即冬季的平均气温比往年低约1 ℃,而该年PAHs的排放量呈现一个明显的峰值;2013年安徽省经历了一个“热夏”,即夏季的平均气温比往年高约0.7 ℃,而该年PAHs的排放量也呈现一个明显的峰值。因此,气温的升高或降低会导致居民用电量的变化(如极冷或极热气候下,空调使用量会显著增加),从而可能导致煤炭消费量的增加和随之带来的PAHs排放量的增加。
2.5 不确定性讨论
燃煤电厂PAHs排放清单估算过程中,锅炉类型、机组容量、年龄、年燃煤量来自安徽省环保局。PAHs的排放因子基于前期机理研究获得[6,16-18,21-22]。燃煤电厂大气大气污染控制设施和条件来源于安徽省环保局和实地调研,均可降低结果的不确定性。但2010—2016年间新建和退役锅炉的年燃煤量基于外推法估算得出。因此,蒙特卡洛计算得到2017年PAHs排放量的不确定度为-25%—26%,而2010—2016年PAHs历史排放量的不确定度为-39%—40%。“关闭小锅炉”导致PAHs减少的排放量的不确定度为-58%—59%。
3. 结论(Conclusion)
(1)安徽省燃煤电厂PAHs的排放量从2010年的15200 kg增加至2017年的16400 kg。8年间累计排放量为126000 kg。
(2)从锅炉年龄来看,这些大机组锅炉和小机组锅炉分别是0—15年和>16年的锅炉的主要贡献者。大锅炉PAHs的排放率和排放强度比其它锅炉要低,主要是其更好的燃烧条件和WFGDs装配率。
(3)与超低排放改造带来的PAHs减排量相比,淘汰小锅炉带来的PAHs减排量更大。
(4)从燃煤电厂PAHs的历史排放量变化来看,区域气温变化(更热的夏天和更冷的冬天)导致了空调用电量的增加和随着而来PAHs排放量的增加。
-
图 5 焦磷酸与镍复合体系中ACS1.5对Ni(Ⅱ)、PP(a)和分解后各主导污染物形态(b)的表观动力学曲线注:图中实线和虚线分别为准一级和准二级动力学方程拟合曲线
Figure 5. The apparent adsorption kinetic curves of the total Ni(Ⅱ)/PP (a) and each kind of main species (b) Note: Solid lines and dash lines were the fitted curves based on pseudo-second-order and pseudo-first-order kinetic equations, respectively.
表 1 焦磷酸络合体系中镍和焦磷酸在ACS1.5上的吸附等温线拟合参数
Table 1. Fitting parameters for the adsorption isotherm of Ni(Ⅱ) and PP onto ACS1.5 from the binary system
目标污染物 Targets Langmuir constants Freundlich constants Qm b R2 Kf n R2 Ni(Ⅱ) 1.466 5.122 0.963 1.292 2.207 0.996 PP 0.834 3.362 0.988 0.599 3.349 0.995 注:C0和Ce分别为初始浓度和平衡浓度(mmol·L−1),Qmax为拟合最大饱和吸附量(mmol·g−1),b和Kf分别是对应模型的拟合系数,代表亲和力的大小,n是吸附优惠性指征. Note: C0 and Ce are the initial and equilibrium concentrations of pollutants (mmol·L−1), respectively. Qmax is the fitted maximum saturation adsorption amount (mmol·g−1). b and Kf are the parameters related to the adsorption affinity in the corresponding isotherm models. n is the index for adsorption preference. 表 2 ACS1.5对焦磷酸-镍复合体系中镍和PP及其主要形态的吸附动力学模型拟合参数
Table 2. Kinetic parameters for the adsorption of total Ni(Ⅱ)/PP and various main species by ACS1.5.
吸附体系Adsorption systems 污染物Species 准一级动力学方程Pseudo-first-order kinetic equation 准二级动力学方程Pseudo-second-order kinetic equation Qe, fit k1 R2 Qe, fit k2 h R2 体系1System 1 Ni(Ⅱ) 0.548 1.89×10−3 0.973 0.652 0.003 1.40×10−3 0.984 PP 0.434 1.42×10−3 0.993 0.549 0.002 7.51×10−4 0.997 体系2System 2 Ni(Ⅱ) 0.551 2.01×10−3 0.969 0.651 0.004 1.51×10−3 0.985 PP 0.825 2.65×10−3 0.965 0.948 0.003 3.04×10−3 0.953 体系1System 1 Ni2+ 0.023 5.59×10−3 0.878 0.025 0.339 2.04×10−4 0.921 NiHP2O7− 0.022 7.15×10−3 0.840 0.024 0.487 2.76×10−4 0.907 NiP2O72- 0.494 1.65×10−3 0.986 0.604 0.003 1.05×10−3 0.993 HP2O73- 0.129 4.98×10−3 0.845 0.142 0.047 9.55×10−4 0.823 注:Qe, fit为模型拟合的平衡吸附量(mmol/g),k1和k2分别是相应的吸附速率常数。h=k2Qe,fit2定义为起始速率常数. Note: Qe, fit is the fitted equilibrium adsorption amount (mmol/g). k1 and k2 are the fitted adsorption rate constants corresponding to the two kinetic models. h=k2Qe,fit2 is defined as the initial adsorption rate constant. -
[1] 石泰山. 电镀废水中的配位剂及其处理 [J]. 电镀与涂饰, 2015, 34(8): 462-465. doi: 10.3969/j.issn.1004-227X.2015.08.011 SHI T S. Complexing agents in electroplating wastewater and their treatments [J]. Electroplating & Finishing, 2015, 34(8): 462-465(in Chinese). doi: 10.3969/j.issn.1004-227X.2015.08.011
[2] SHAN C, YANG B W, XIN B, et al. Molecular identification guided process design for advanced treatment of electroless nickel plating effluent [J]. Water Research, 2020, 168: 115211. doi: 10.1016/j.watres.2019.115211 [3] YUAN Y, ZHAO W, LIU Z C, et al. Low-Fe(Ⅲ) driven UV/Air process for enhanced recovery of heavy metals from EDTA complexed system [J]. Water Research, 2020, 171: 115375. doi: 10.1016/j.watres.2019.115375 [4] LI Q M, SONG H O, HAN R M, et al. Efficient removal of Cu(Ⅱ) and citrate complexes by combined permanent magnetic resin and its mechanistic insights [J]. Chemical Engineering Journal, 2019, 366: 1-10. doi: 10.1016/j.cej.2019.02.070 [5] 廖文. 络合剂对阳离子在EDI中迁移的影响与多级回用的研究[D]. 杭州: 浙江大学, 2013. LIAO W. Influence of migration of the metal ions under the exiting of chelating agent in EDI [D]. Hangzhou: Zhejiang University, 2013(in Chinese).
[6] REPO E, WARCHOŁ J K, BHATNAGAR A, et al. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water [J]. Water Research, 2013, 47(14): 4812-4832. doi: 10.1016/j.watres.2013.06.020 [7] 郁佳程, 杨璐阳, 王励珽, 等. 基于聚多巴胺的环境功能材料吸附水体重金属的研究进展 [J]. 环境化学, 40(7): 2204-2216. YU J C, YANG L Y, WANG L T, et al. Research progress of polydopamine−based environmental functional materials for the adsorption of the heavy metals in water [J]. Environmental Chemistry, 2021, 40(7): 2204-2216 (in Chinese).
[8] LING C, REN Z X, WEI M M, et al. Highly selective removal of Ni(Ⅱ) from plating rinsing wastewaters containing [Ni-xNH3-yP2O7]n complexes using N-chelating resins [J]. Journal of Hazardous Materials, 2020, 398: 122960. doi: 10.1016/j.jhazmat.2020.122960 [9] 韦蒙蒙, 刘福强, 凌晨, 等. 常见有机酸对多胺树脂吸附镍离子的影响特性 [J]. 离子交换与吸附, 2015, 31(3): 212-220. WEI M M, LIU F Q, LING C, et al. Effect of some common organic chelating acids on adsorption of nickel ions by polyamine resin [J]. Ion Exchange and Adsorption, 2015, 31(3): 212-220(in Chinese).
[10] LING C, ZHAO Y X, REN Z X, et al. Synergistic co-removal of zinc(Ⅱ) and cefazolin by a Fe/amine-modified chitosan composite [J]. Chinese Chemical Letters, 2019, 30(12): 2196-2200. doi: 10.1016/j.cclet.2019.09.035 [11] 凌晨, 刘福强, 韦蒙蒙, 等. Cu(Ⅱ)、Ca(Ⅱ)共存对IRC748树脂吸附四环素的影响与机制 [J]. 环境化学, 2016, 35(5): 884-892. doi: 10.7524/j.issn.0254-6108.2016.05.2015112504 LING C, LIU F Q, WEI M M, et al. Effect of co-existing Cu(Ⅱ)/Ca(Ⅱ) on the adsorption of tetracycline onto IRC748 resin [J]. Environmental Chemistry, 2016, 35(5): 884-892(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.05.2015112504
[12] 方景礼. 电镀配合物--理论与应用(精) [M]. 北京: 化学工业出版社, 2008. FANG J L. Theory & application of coordination compounds in electroplating[M]. Beijing: Chemical Industry Press, 2008 (in Chinese).
[13] MAKETON W, ZENNER C Z, OGDEN K L. Removal efficiency and binding mechanisms of copper and copper−EDTA complexes using polyethyleneimine [J]. Environmental Science & Technology, 2008, 42(6): 2124-2129. [14] LING C, LIU F Q, PEI Z G, et al. Citric acid enhanced copper removal by a novel multi-amines decorated resin [J]. Scientific Reports, 2015, 5: 9944. doi: 10.1038/srep09944 [15] ZHANG Y H, ZHU C Q, LIU F Q, et al. Effects of ionic strength on removal of toxic pollutants from aqueous media with multifarious adsorbents: A review [J]. Science of the Total Environment, 2019, 646: 265-279. doi: 10.1016/j.scitotenv.2018.07.279 [16] FU L C, LIU F Q, MA Y, et al. High-efficient technique to simultaneous removal of Cu(Ⅱ), Ni(Ⅱ) and tannic acid with magnetic resins: Complex mechanism behind integrative application [J]. Chemical Engineering Journal, 2015, 263: 83-91. doi: 10.1016/j.cej.2014.11.041 [17] ZHAO W, LIU Z C, YUAN Y, et al. Insight into Cu(II) adsorption on polyamine resin in the presence of HEDP by tracking the evolution of amino groups and Cu(II)-HEDP complexes [J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 5256-5263. [18] PAN B, QIU M Y, WU M, et al. The opposite impacts of Cu and Mg cations on dissolved organic matter-ofloxacin interaction [J]. Environmental Pollution, 2012, 161: 76-82. doi: 10.1016/j.envpol.2011.09.040 [19] WANG Q Y, TIAN Y, KONG L C, et al. A novel 3D superelastic polyethyleneimine functionalized chitosan aerogels for selective removal of Cr(VI) from aqueous solution: Performance and mechanisms [J]. Chemical Engineering Journal, 2021, 425: 131722. doi: 10.1016/j.cej.2021.131722 [20] LIU C K, BAI R B, SAN L Y Q. Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms [J]. Water Research, 2008, 42(6/7): 1511-1522. -






 DownLoad:
DownLoad: