纳米零价铁的表征及改性研究进展
Research progress on the characterization and modification of nanoscale zero-valent iron for applications
-
摘要: 纳米零价铁(nanoscale zero-valent iron,nZVI)因其在地下水处理和土壤修复中具有应用前景一直广受关注.但在实际应用中,纳米零价铁nZVI依旧存在颗粒易团聚、传输能力弱和污染物难以选择性去除等方面的问题.为突破这些应用瓶颈,目前已开展了大量nZVI改性工作,并对污染物去除机理进行了深入研究.相关研究很大程度上解决了目前nZVI在环境应用中存在的缺陷,大幅提升了nZVI的环境修复效率.本文归纳了近年来nZVI颗粒在机理研究和性能优化中涉及的表征方法,详细介绍了nZVI表面改性技术(金属掺杂、表面涂层、乳化和负载等)及其优势.同时,展望了nZVI在实际应用中面临的科学挑战与发展机遇,其有助于学者们对nZVI的相关应用有更深的理解,有望为nZVI等环境纳米材料实际应用提供相关借鉴.Abstract: Nanoscale zero-valent iron(nZVI) has received considerable attention due to its potential applications in groundwater treatment and site remediation. The current field application of nZVI is constrained by the problems of agglomeration of particles, poor transmission efficiency, and low selectivity of pollutants. Considering the above-mentioned bottlenecks, the research on surface modification is expected to improve the dispersibility, mechanical strength and reactivity of nZVI, thus greatly improving the environmental remediation efficiency. This article reviews the recent research progress of nZVI characterization methods to accurately reveal the mechanisms on its interaction with pollutants, and summarizes the nZVI surface modification technologies (including metal doping, surface coating, emulsification and supporting). Furthermore, the scientific challenges and future development opportunities of nZVI in practical application are also prospected.
-
“双碳”背景下,绿色低碳的污水处理技术成为发展重点。好氧颗粒污泥 (aerobic granular sludge,AGS) 是微生物自凝聚形成的颗粒状活性污泥,具有沉降性能好、生物量高、可同步去除碳氮磷等优点,而相比于传统活性污泥工艺,AGS能节省50%~75%的占地面积、20%~25%的运行费用和23%~40%的电耗,故该技术符合当前减污降碳的发展目标,具有一定应用前景[1]。世界范围内已有80余座污水处理厂在序批式反应器 (sequencing batch reactor,SBR) 中成功应用了AGS技术,但在连续流反应器中成功应用AGS技术的仅10余座,连续流AGS的推广应用还未取得实质性的突破[2]。尽管SBR更易于培养AGS,但存在处理量小、设备使用率低等缺陷。而连续流是现有污水处理厂的主要运行模式,故连续流AGS的培养备受关注。
丰盛-饥饿条件[3-4]、基于污泥沉降速度[5-6]、尺寸或密度[7-8]的选择压及水力剪切力[9]等被认为是SBR-AGS颗粒化的关键影响因素。但连续流的培养环境与SBR截然不同,这些关键影响因素更难实现。SUN等[10]采用系列串联的完全混合反应器组成整体推流的连续流系统,同时沉淀池采用1 min进水→4 min静态沉淀→1 min排水的间歇运行模式,创造基于沉降速度的选择压,研究了丰盛-饥饿条件对连续流AGS形成的必要性。LIU等[11-12]应用双区沉淀池,通过调整沉淀区上方挡板的高度设置污泥选择压,在AAO系统中培养出平均粒径为210 µm的AGS。以上研究证实了丰盛-饥饿条件和选择压在连续流AGS培养中的必要性和可行性,但沉淀池的运行策略仍较复杂。在厌氧颗粒污泥的研究中,顶部为三相分离器的升流式反应器[13-14]能很好地富集颗粒污泥,但这些研究大多采用大高径比的柱式反应器,与现有污水处理厂的平铺式构筑物不兼容,开发的培养策略难以直接应用。再加上现有研究多为接种成熟AGS的小试实验,缺乏直接在连续流模式下培养AGS的更大尺度研究。
基于此,本团队提出一种新型的连续流AGS反应器,将三相分离器与传统活性污泥工艺组合,构成反应耦合沉淀一体式的反应器,以中试尺度在现有污水处理厂进行改造应用。通过向系统中接种活性污泥,以低浓度市政污水为基质,探究AGS的形成过程及其形貌和结构特性,并通过监测中试系统对NH4+-N和TN的去除效果,再结合微生物群落结构角度以探索系统的脱氮机理,以期为连续流AGS这一绿色低碳处理工艺的应用推广提供参考。
1. 材料与方法
1.1 中试系统及运行方法
中试系统位于河北省某市政污水处理厂原厌氧池,日处理量为3 000 m3。该厂出水标准由《城镇污水处理厂污染物排放标准》 (GB18918-2002) 一级A改为执行地方标准,其中COD、NH4+-N和TP的排放浓度限值分别由50、5 (8) 和0.5 mg·L−1降为40、2.0 (3.5) 和0.4 mg·L−1,SS、BOD5和TN不变。根据该厂2019年的运行数据,提标后现有工艺出水NH4+-N和TN存在超标风险。
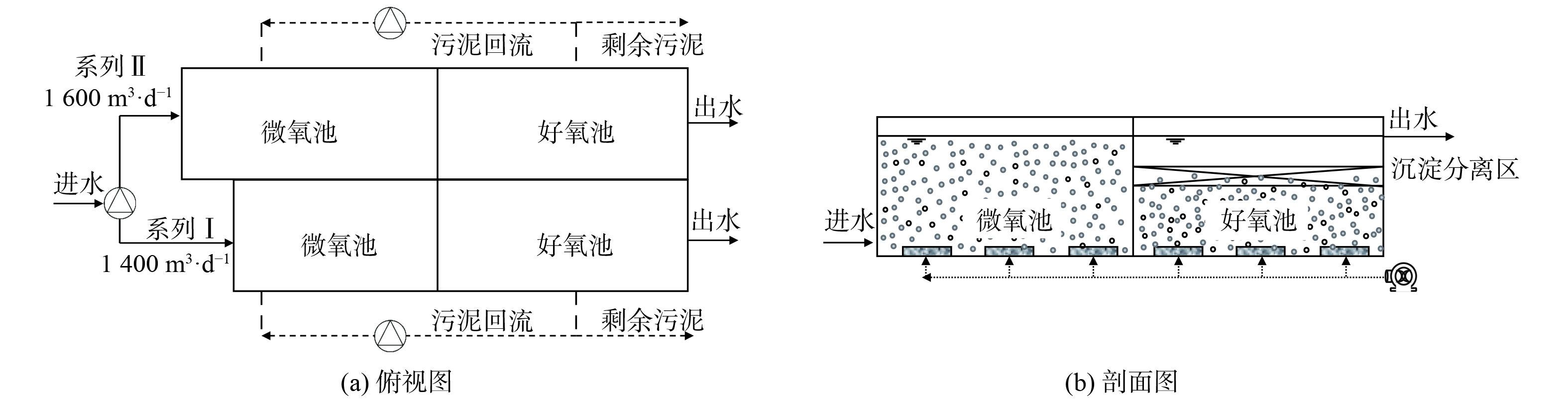
中试系统由原厌氧池改造而来 (图1) ,该系统分为I和II两个系列,均由微氧池、好氧池及置于好氧池内部、基于三相分离器的沉淀分离装置组成。系列I:微氧池11.0 m×6.0 m×6.5 m,好氧池13.5 m×6.0 m×3.8 m,好氧池内沉淀分离装置13.5 m×6.0 m×1.0 m;系列II:微氧池13.8.0 m×6.0 m×6.5 m,好氧池13.5 m×6.0 m×3.8 m,好氧池内沉淀分离装置13.5 m×6.0 m×1.0 m。进水流量、污泥回流量、剩余污泥外排量及曝气量均采用变频控制器控制。污泥质量浓度保持在4~7 g·L−1,采用气提回流控制污泥回流比约200%,每日排泥控制污泥龄26~30 d,调整曝气量使得微氧池溶解氧 (DO) 为0.2~0.5 mg·L−1,好氧池DO为1.0~3.0 mg·L−1。与传统活性污泥工艺相比,中试系统有如下特点:1) 未设缺氧池,前置的反应器为微氧池,旨在通过控制微量曝气以充分利用原水中的碳源实现同步硝化反硝化脱氮;2) 通过好氧池内置的沉淀分离装置完成固-液-气三相分离,省去二沉池,减少占地面积,同时内、外回流合二为一,可降低运行能耗。
1.2 原水水质及接种污泥
中试系统进水为该污水处理厂旋流沉砂池出水,其水质指标如下:COD (236.5±51.6) mg·L−1,NH4+-N (57.3±14.6) mg·L−1,TN (67.2±14.7) mg·L−1,TP (6.0±1.7) mg·L−1。原水C/N仅为3.6,生物脱氮碳源不足,故在微氧池定量投加乙酸钠作为外加碳源。这使得实际进水COD为 (328.1±73.6) mg·L−1,C/N为4.9。
中试系统接种该厂生化池活性污泥,其混合液悬浮固体浓度 (MLSS) 为4.2 g·L−1,挥发性悬浮固体浓度 (MLVSS) 为1.9 g·L−1,污泥指数 (SVI30) 为59.4 mL·g−1,粒径分布 (D50) 为28.9 μm,是典型的絮状活性污泥。
1.3 分析与检测方法
中试系统于2021年1月24日完成改造和接种工作,进出水采样时间为4月21日至7月5日。这段时间中试系统处于稳定运行状态,出水水质足以评估中试系统的污染物去除效果。采用纳氏分光光度法测定进出水NH4+-N;采用碱性过硫酸钾消解紫外分光光度法测定TN;DO和pH采用便携式测定仪 (HACH,HQ30d,美国) 测定。
采用激光粒度仪 (Beckman,LS13320,美国) 测定接种污泥的粒径分布,并于7月13日和9月13日在中试系统中进行采样。采用数码相机观察AGS的宏观形态,通过扫描电子显微镜 (SEM) 观察AGS的微观结构及微生物形态。SEM样品制备过程[15]:用4%戊二醛固定;再用去离子水多次浸泡冲洗;经50%、70%、85%、95%、100%乙醇梯度脱水;临界点干燥、粘样、镀膜观察。通过流变仪 (Aaton Paar Physica MCR301,奥地利) 测定动态粘弹性。并分析了中试AGS与接种污泥的流变特性差异。具体步骤如下:采用平板-平板测量系统,平板直径50 mm,用一次性滴管滴加样品,刮除平板外多余样品后,在固定角频率5 rad·s−1、温度25 ℃条件下测定[16]。此外,为探究AGS中微生物群落结构的变化,使用孔径0.2 mm的筛网收集中试中粒径<0.2 mm (命名为F) 和>0.2 mm (命名为G) 及接种污泥样品 (命名为AS) 委托上海美吉生物医药科技有限公司进行16S rRNA高通量测序及种群分析。
2. 结果与讨论
2.1 污泥形态与粒径变化
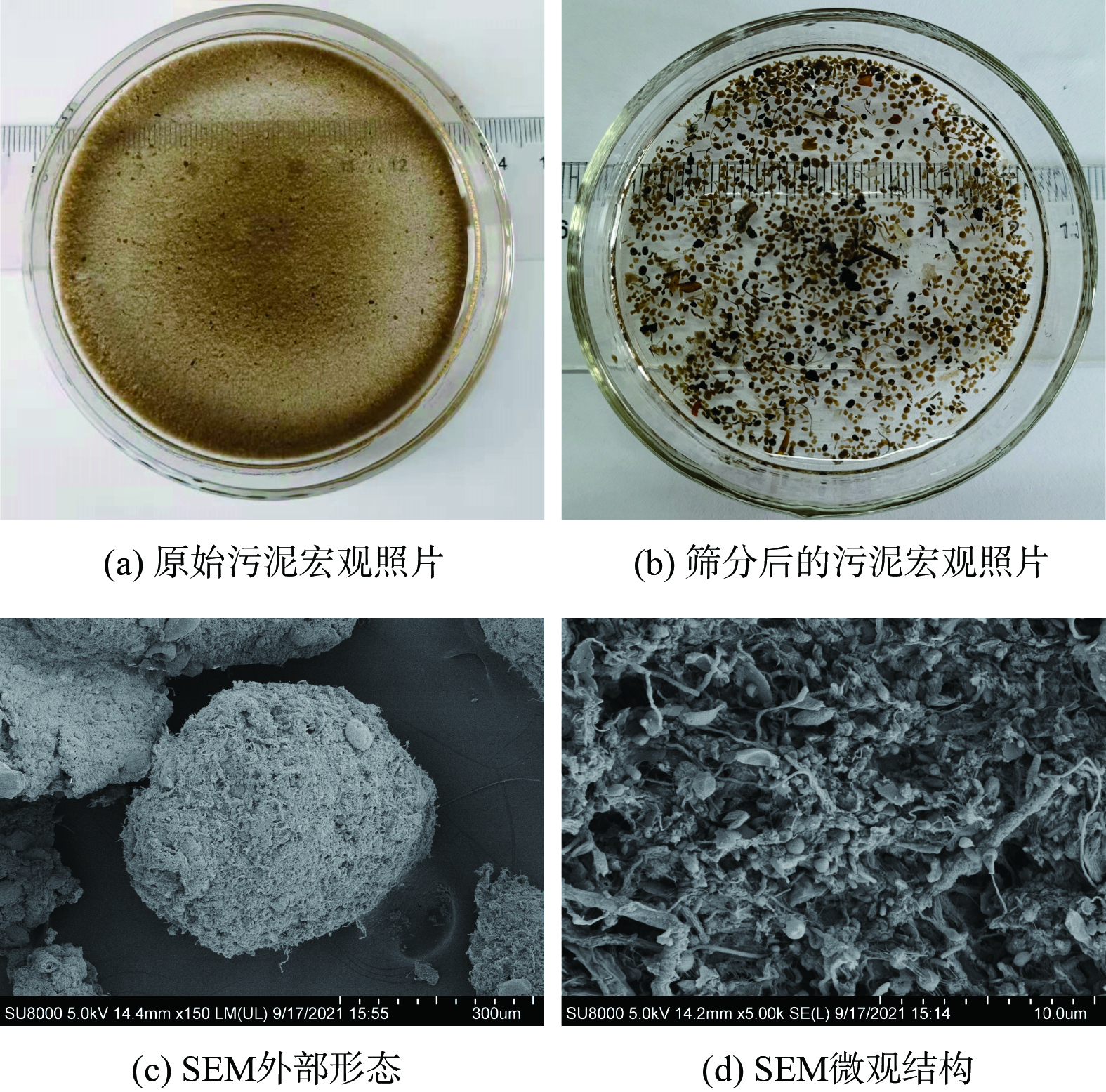
图2 (a) 表明,在宏观上中试系统好氧池中的原始污泥与接种污泥几乎没有差别,但中试系统中的污泥经孔径为0.2 mm的筛网筛分后可得到粒径较大的AGS,采用数码相机和SEM观察AGS的形貌,结果如图2 (b)~(d) 所示。中试系统筛分得到的AGS轮廓清晰、紧致饱满,为形状规则的球形和椭球形。SEM图像表明AGS微观上主要由球状菌紧密相连,表面有少量丝状菌,这与文献[17]报道的实验室培养AGS形貌非常相似。这表明中试系统在连续流模式下,接种絮状活性污泥后使用实际低浓度低C/N的市政污水已成功培养出了AGS。
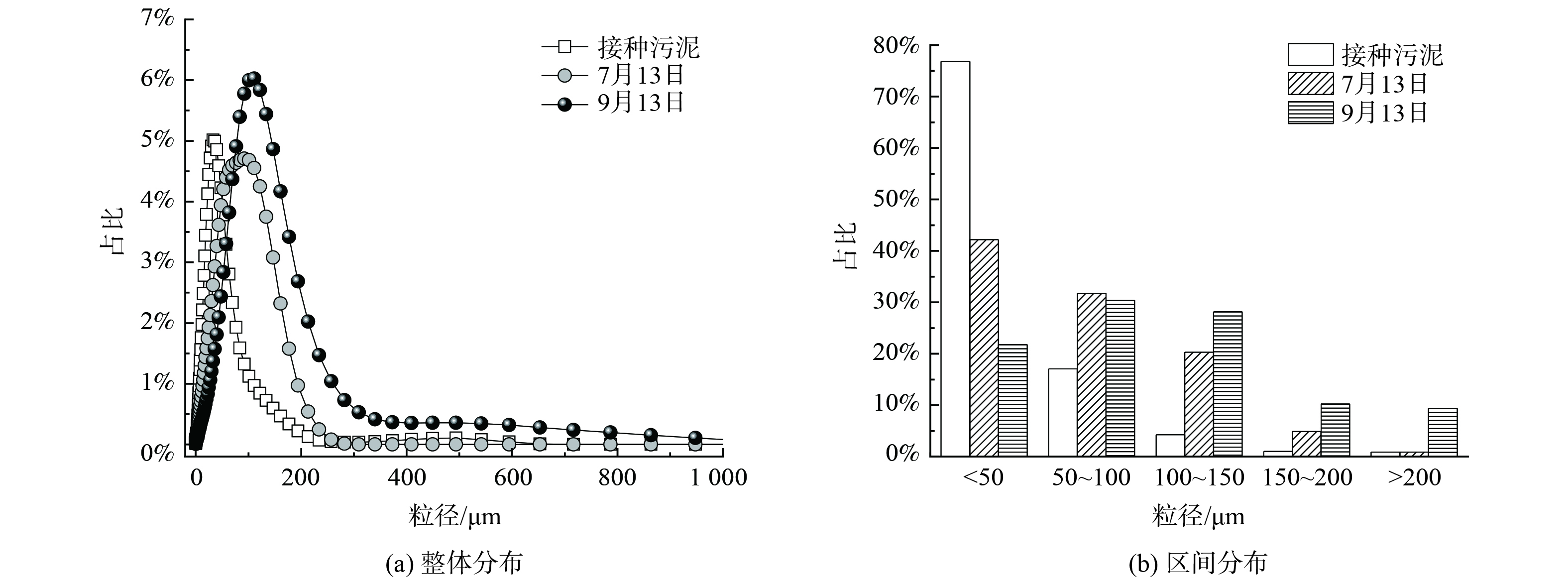
图3为接种污泥及中试系统稳定运行过程中污泥粒径分布的变化情况。粒径分布曲线右移表明随着中试系统的运行,污泥粒径逐渐增大。接种污泥的D50为28.9 μm。到了7月13日,中试系统中污泥D50为57.8 μm;到了9月13日,D50进一步增大到90.1 μm,较接种污泥粒径增大了3.1倍。粒径区间分布发现,接种污泥粒径主要分布在<50 μm,其占比高达76.8%,少量污泥粒径分布在50~100 μm,占比17.1%。但到7月13日,中试系统中污泥粒径较均匀地分布到了<50 μm、50~100 μm及100~150 μm,占比分别为42.2%、31.7%及20.3%。粒径为150~200 μm的污泥占比由初始的1.0%增至4.9%。到了9月13日,更是增加到10.3%。而粒径>200 μm的污泥占比由初始的0.9%增至9.4%,实现了数量级跃增。连续流模式下培养的AGS粒径小于SBR中培养的AGS粒径[18],这是由于连续流系统往往处于完全混合的状态,反应器内的基质浓度与出水浓度相当,更低的基质浓度意味着更小的扩散内径,从而限制了连续流中AGS的粒径。此外,本研究中污泥的颗粒化比例 (粒径>200 μm的污泥占比) 低于文献[12, 19]的报道值。这是由于中试系统中没有设置污泥选择压,系统中形成的AGS与絮状污泥一起作为剩余污泥被排出,不利于AGS的富集,从而导致颗粒化比例较低。
2.2 污泥流变特性
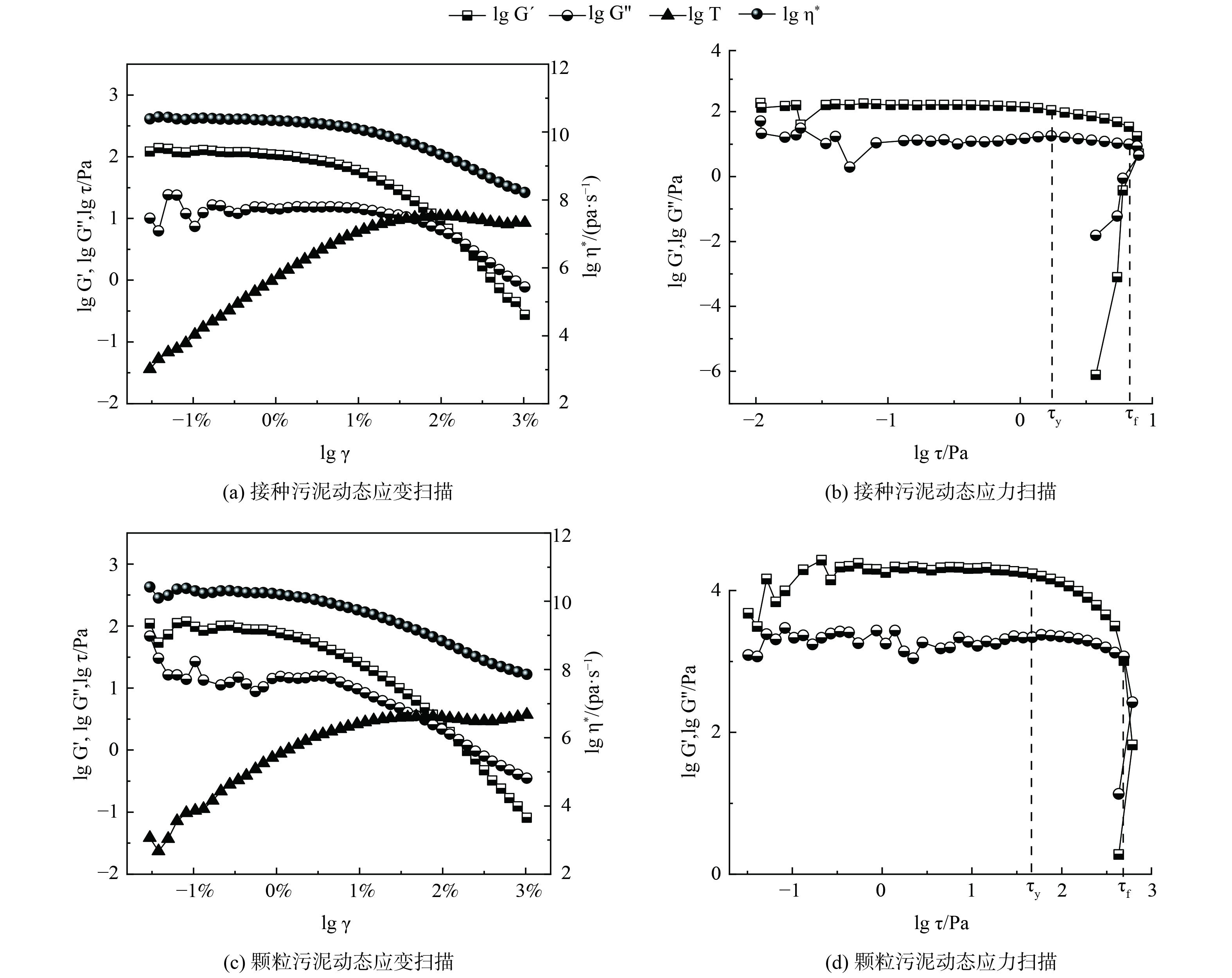
接种污泥及中试系统培养的AGS的流变特性如图4所示。通过动态应变扫描确定污泥的线性黏弹区,接种污泥和AGS的临界应变点均在1%左右。当应变超过线性黏弹区后,储能模量G´开始下降,这表明污泥样品的结构开始被破坏。2份污泥样品的G´均大于G´´,说明污泥样品具有凝胶状或固体状的结构,可被称为黏弹性固体。此外,由实验数据可得到污泥样品的屈服应力τy,即黏弹性极限处的剪切应力和流动应力τf,即样品变形过程G´=G´´处的剪切应力。接种污泥和AGS的τy分别为2.2和37.3 Pa,τf分别为7.9和390.3 Pa。这说明中试系统培养的AGS机械强度远高于接种的活性污泥。这主要是由于AGS中胞外多聚物 (extracellular polymeric substance,EPS) 的含量往往高于活性污泥,EPS将AGS中的微生物团聚在一起,从而使得AGS比絮状污泥有更高的机械强度。
2.3 污染物去除效果
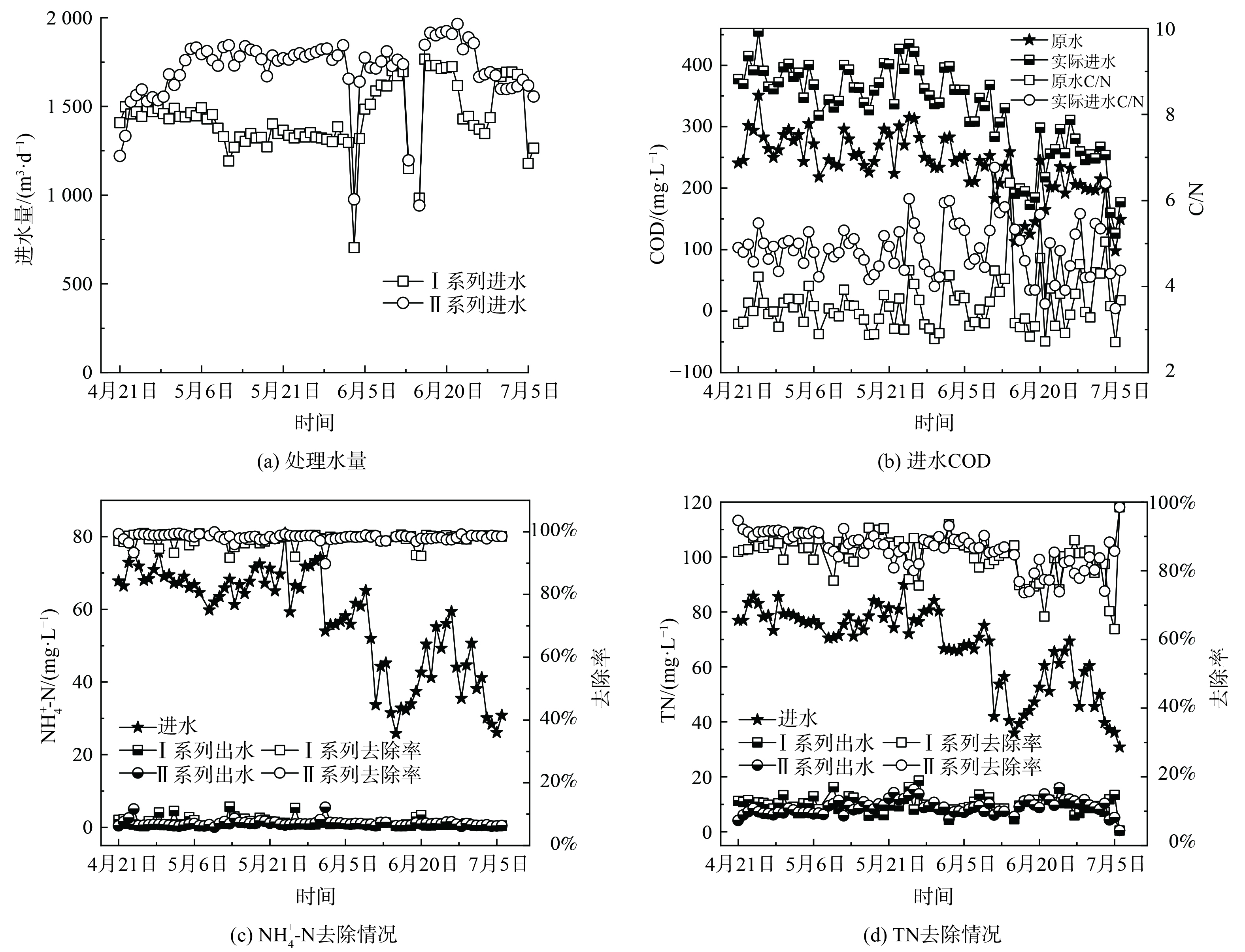
中试系统处理水量情况如图5 (a) 所示。除6月3日、6月14日和15日由于进水泵故障,其余时间中试系统处理量均略高于设计值,I、II系列平均日处理量分别为1 454.9和1 722.4 m3·d−1。由图5 (b) 可知,原水COD呈现出明显的雨季降低的变化趋势,在进入雨季前 (4—5月) ,原水COD稳定在 (267.7±30.2) mg·L−1,而进入雨季 (6—7月) 后,平均COD降至 (200.1±54.9) mg·L−1。这是由于尽管该厂收水区域的排水体制为分流制,但可能存在管网的错接、混接等问题,导致雨季部分雨水进入污水管网,从而使得污水处理厂进水COD偏低。原水C/N为 (3.6±0.6) ,属于典型的低C/N污水,生物脱氮难度大。因此,需要定量投加乙酸钠作为碳源,而保证进水COD达到 (328.1±73.6) mg·L−1,C/N达 (4.9±0.6) 。
中试系统对NH4+-N和TN的去除情况如图5 (c) 和 (d) 所示。与进水COD类似,进水NH4+-N和TN在雨季呈现出明显降低的趋势。在进入雨季前,进水NH4+-N和TN分别为 (68.5±4.5) mg·L−1和 (78.2±4.5) mg·L−1;在进入雨季后,分别降为 (45.9±14.7) mg·L−1和 (55.6±15.7) mg·L−1。I系列和II系列出水NH4+-N分别为 (1.3±1.1) mg·L−1和 (1.0±0.8) mg·L−1,平均去除率分别为97.7%和98.2%。出水NH4+-N基本保持在2 mg·L−1以下,满足地方标准要求的2.0 mg·L−1,达标时间占比分别为81.8%和93.5%。其中,I系列由于曝气设备故障导致超标时间多于II系列。出水TN分别为 (9.9±2.8) mg·L−1和 (9.1±2.6) mg·L−1,平均去除率分别为84.8%和85.7%。出水TN基本保持在10 mg·L−1左右,满足地方标准要求的15.0 mg·L−1,达标时间占比分别为94.8%和96.1%。此外,中试系统不仅获得了良好的出水水质,而且由于二沉池耦合在好氧池内部,省去了二沉池,可减小占地面积,同时硝化液回流和污泥回流合二为一,能降低系统的运行能耗。
2.4 微生物群落结构分析
为探究中试系统中污泥微生物群落结构的变化,使用孔径0.2 mm的筛网收集粒径<0.2 mm (F) 和>0.2 mm (G) 及接种污泥样品 (AS) 进行高通量测序。如表1所示,Coverage指数均大于99%,表明测序的结果具有代表性。Alpha多样性分析中的Simpson和Shannon指数均用于反映群落多样性;Simpson指数越大说明群落多样性越低;而Shannon值越大说明群落多样性越高。中试系统中F和G的Simpson指数均大于AS的,而Shannon值则均小于AS的。这表明中试系统污泥群落多样性减少,这可能与中试系统条件下能选择性富集功能微生物有关。
表 1 样本Alpha多样性指数Table 1. Alpha-diversity of the samples样本 Simpson Shannon Coverage AS 0.013 5.5 99.3% F 0.059 4.7 99.2% G 0.060 4.9 99.3% 微生物群落结构组成分析如图6所示。在门水平上,AS、F和G中优势菌群类似,均为Proteobacteria (34.45%、46.53%和50.51%),Chloroflexi (17.36%,19.74%和16.58%) 和Bacteroidota (15.03%,9.92%和13.00%) 。这3类微生物在营养物的去除中具有重要作用[20],是污水处理中常见的微生物。除这3类优势菌群外,其余菌群丰度也发生了较大变化,如Actinobacteriota在AS中的相对丰度为13.08%,但在F中为6.37%,在G仅为4.39%;类似的还有Patescibacteria,其在AS中的相对丰度为5.27%,但在F和G的相对丰度仅为1.56%和1.19%;而Firmicutes在AS中的相对丰度为3.11%,尽管在F中增加到了6.26%,但在G中仅为2.30%。这表明相比于接种污泥,中试系统中的污泥微生物群落结构发生了变化,而且粒径<0.2 mm的污泥与粒径>0.2 mm的污泥微生物群落结构也有显著的差异。在属水平上,接种污泥中的优势属为Methylophilaceae (6.10%) ,Microtrichales (5.28%) ,Methylotenera (4.81%) ,Saprospiraceae (4.28%) 和Saccharimonadales (4.30%) 。而在中试系统中,Methylophilaceae和Methylotenera得到了显著富集,F和G中的丰度分别为23.73%和6.34%,24.61%和7.16%,丰度和为AS的3倍以上。Methylophilaceae和Methylotenera普遍存在于自然环境中,包括淡水、土壤、污水等生态位,是一种兼性厌氧、以甲醇等为生长基质的甲基营养型细菌,因其能在有氧条件下进行反硝化而受到关注[21]。中试系统由微氧池和好氧池组成,而没有设置缺氧池,NH4+-N在微氧池或好氧池中被氧化,同时生成的NO2−-N/NO3−-N的也只能在微氧池或好氧池被还原成N2,这可能是中试系统大量富集好氧反硝化菌Methylophilaceae和Methylotenera的原因。同时,异养菌Microtrichales和Saccharimonadales的丰度则显著下降,由AS中的5.28%和4.30%分别降低至1.80%和1.11%,1.09%和0.57%,这可能与系统中有机物利用的途径有关。
2.5 颗粒化及脱氮机理分析
新型微氧-好氧耦合沉淀一体式反应器构型对AGS的形成、系统脱氮性能及微生物群落结构变化有着重要影响。在好氧池内,底部曝气和微氧池出水 (即好氧池进水) 产生推动力,使得好氧池内部混合液向上流动,与内置的三相分离器碰撞并实现固液气三相分离。分离的气体经三相分离器间的气-液平面逸散到空气中,液体经出水渠排出系统,而泥水混合液只能向下继续流动,在好氧区与三相分离器间形成剧烈的内循环流动,为AGS的形成提供关键的驱动力-水力剪切力。水力剪切力在颗粒化初期能促进微生物的随机运动,增加微生物间的有效碰撞,有利于形成初始可逆的微生物聚集体[22]。诱导EPS的分泌可增强细胞表面的疏水性,增加聚集体的密度,从而进一步形成不可逆的微生物聚集体[23]。在稳定阶段,水力剪切力不断剥离成熟AGS表面附着生长的丝状菌,维持AGS的形貌和优势地位[5]。这与DAI 等[24]的发现类似,即由反应器内部纵向循环产生水力剪切力培养AGS的机理。此外,沉淀耦合在好氧池内部,省去了二沉池,可节省占地面积,同时将内、外回流合二为一,降低运行能耗。
在微氧池中,DO控制在0.2~0.5 mg L−1,当微氧池COD为50~100 mg L−1时,DO可渗透AGS外表层10~18 µm处[25],故粒径大于20~36 µm的AGS内可形成外层好氧、内层缺氧/厌氧的分层结构。进水中的NH4+-N在外层好氧条件下被氧化成NOx−-N,生成的NOx−-N扩散至内层,在内层缺氧/厌氧的条件下发生反硝化,使得系统可在微氧池中完成生物脱氮。但这一脱氮途径只存在于AGS形成后。在启动初期,接种的絮状污泥无法通过该途径完成生物脱氮,系统脱氮性能较差;在启动中期,随着中试系统的运行,尽管AGS还未形成,但系统富集了大量的好氧反硝化菌Methylophilaceae和Methylotenera,能通过好氧反硝化途径完成脱氮;而在AGS形成后的稳定运行阶段,两种脱氮途径共同完成生物脱氮过程。此外,微氧池中的反硝化过程不仅能充分利用原水中的碳源,还能降低进入好氧池中的有机物浓度,创造微氧池丰盛-好氧池饥饿的运行条件,有利于AGS的长期稳定性。
3. 结论
1) 以低浓度市政污水为基质、接种活性污泥,在中试规模 (3 000 m3 d−1) 的连续流运行模式下,可培养出长期稳定存在的AGS,平均粒径由接种污泥的28.9 μm增至90.1 μm,其中粒径>100 μm的占47.8%,>200 μm的占9.4%。
2) 中试系统I、II系列出水NH4+-N分别为 (1.3±1.1) mg·L−1和 (1.0±0.8) mg·L−1,出水TN分别为 (9.9±2.8) mg·L−1和 (9.1±2.6) mg·L−1,系统具有良好的脱氮效果,能满足该厂的提标改造要求。
3) 中试系统大量富集了好氧反硝化菌Methylophilaceae和Methylotenera,好氧反硝化途径可能在脱氮中起重要作用。微氧池中的反硝化过程能充分利用原水碳源,降低进水有机物浓度,有利于维持系统的稳定高效低碳运行。
-
[1] WANG C B,ZHANG W X. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs[J]. Environmental Science & Technology, 1997, 31:2154-2156. [2] LI S, WANG W, LIANG F, et al. Heavy metal removal using nanoscale zero-valent iron (nZVI):Theory and application[J]. Journal of Hazardous Materials, 2017, 322:163-171. [3] MUELLER N C, BRAUN J, BRUNS J, et al. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe[J]. Environmental Science and Pollution Research, 2012, 19:550-558. [4] WEI Y T, WU S C, CHOU C M, et al. Influence of nanoscale zero-valent iron on geochemical properties of groundwater and vinyl chloride degradation:A field case study[J]. Water Research, 2010, 44:131-140. [5] FU F, DIONYSIOU D D,LIU H. The use of zero-valent iron for groundwater remediation and wastewater treatment:A review[J]. Journal of Hazardous Materials, 2014, 267:194-205. [6] SIGNORINI L, PASQUINI L, SAVINI L, et al. Size-dependent oxidation in iron/iron oxide core-shell nanoparticles[J]. Physical Review B, 2003, 68:195423. [7] WANG C, BAER D R, AMONETTE J E, et al. Morphology and electronic structure of the oxide shell on the surface of iron nanoparticles[J]. Journal of the American Chemical Society, 2009, 131:8824-8832. [8] YAN W, VASIC R, FRENKEL A I, et al. Intraparticle reduction of arsenite (As(Ⅲ)) by nanoscale zerovalent iron (nZVI) investigated with In situ X-ray absorption spectroscopy[J]. Environmental Science & Technology, 2012, 46:7018-7026. [9] YAN W, HERZING A A, KIELY C J, et al. Nanoscale zero-valent iron (nZVI):Aspects of the core-shell structure and reactions with inorganic species in water[J]. Journal of Contaminant Hydrology, 2010, 118:96-104. [10] 黄潇月, 王伟, 凌岚, 等.纳米零价铁与重金属的反应:"核-壳"结构在重金属去除中的作用[J]. 化学学报. 2017, 75(6):529-537. HUANG X Y, WANG W, LING L, et al. Heavy metal-nZVI reactions:The core-shell structure and applications for heavy metal treatment[J]. Acta Chim. Sinica, 2017, 75(6):529-537(in Chinese)
[11] LING L, PAN B,ZHANG W X. Removal of selenium from water with nanoscale zero-valent iron:Mechanisms of intraparticle reduction of Se(Ⅳ)[J]. Water Research, 2015, 71:274-281. [12] 李钰婷, 张亚雷, 代朝猛, 等.纳米零价铁颗粒去除水中重金属的研究进展[J]. 环境化学, 2012, 31(9):1349-1354. LI Y T, ZHANG Y L, DAI C M, et al. The advance on removal of heavy metals in water by nanoscale zero-valent iron[J]. Environmental Chemistry, 2012, 31(9):1349-1354(in Chinese).
[13] 夏雪芬, 滑熠龙, 黄潇月, 等.纳米零价铁对水中砷和硒去除的比较研究[J]. 化学学报, 2017, 75(6):594-601. XIA X F, HUA Y L, HUANG X Y, et al. Removal of arsenic and selenium with nanoscale zero-valent iron (nZVI)[J]. Acta Chim Sinica, 2017, 75(6):594-601(in Chinese).
[14] LING L, HUANG X Y,ZHANG W X. Enrichment of precious metals from wastewater with core-shell nanoparticles of iron[J]. Advanced Materials, 2018, 30:1705703. [15] LING L, TANG C,ZHANG W X. Visualization of silver nanoparticle formation on nanoscale zero-valent iron[J]. Environmental Science & Technology Letters, 2018, 5:520-525. [16] LIU Z, DONG S, ZOU D, et al. Electrochemically mediated nitrate reduction on nanoconfined zerovalent iron:Properties and mechanism[J]. Water Research, 2020, 173:115596. [17] KATSOYIANNIS I A, RUETTIMANN T,HUG S J. pH dependence of fenton reagent generation and As(Ⅲ) oxidation and removal by corrosion of zero valent iron in aerated water[J]. Environmental Science & Technology, 2008, 42:7424-7430. [18] SEDLAK D L,ANDREN A W. Oxidation of chlorobenzene with Fenton's reagent[J]. Environmental Science & Technology, 1991, 25:777-782. [19] SHI Z, FAN D, JOHNSON R L, et al. Methods for characterizing the fate and effects of nano zerovalent iron during groundwater remediation[J]. Journal of Contaminant Hydrology, 2015, 181:17-35. [20] SU Y, ADELEYE A S, KELLER A A, et al. Magnetic sulfide-modified nanoscale zerovalent iron (S-nZVI) for dissolved metal ion removal[J]. Water Research, 2015, 74:47-57. [21] SHARD A G. A straightforward method for interpreting XPS data from core-shell nanoparticles[J]. The Journal of Physical Chemistry C, 2012, 116:16806-16813. [22] XI Y, MALLAVARAPU M,NAIDU R. Reduction and adsorption of Pb2+ in aqueous solution by nano-zero-valent iron-A SEM, TEM and XPS study[J]. Materials Research Bulletin, 2010, 45:1361-1367. [23] KUMAR N, AUFFAN M, GATTACCECA J, et al. Molecular insights of oxidation process of iron nanoparticles:Spectroscopic, magnetic, and microscopic evidence[J]. Environmental Science & Technology, 2014, 48:13888-13894. [24] WANG Q, SNYDER S, KIM J, et al. Aqueous ethanol modified nanoscale zerovalent iron in bromate reduction:Synthesis, characterization, and reactivity[J]. Environmental Science & Technology, 2009, 43:3292-3299. [25] YAN W, LIEN H L, KOEL B E, et al. Iron nanoparticles for environmental clean-up:Recent developments and future outlook[J]. Environmental Science:Processes & Impacts, 2013, 15:63-77. [26] WANG C M, BAER D R, THOMAS L E, et al. Void formation during early stages of passivation:Initial oxidation of iron nanoparticles at room temperature[J]. Journal of Applied Physics, 2005, 98:094308. [27] NURMI J T, TRATNYEK P G, SARATHY V, et al. Characterization and properties of metallic iron nanoparticles:Spectroscopy, electrochemistry, and kinetics[J]. Environmental Science & Technology, 2005, 39:1221-1230. [28] LIU Y, CHOI H, DIONYSIOU D, et al. Trichloroethene hydrodechlorination in water by highly disordered monometallic nanoiron[J]. Chemistry of Materials, 2005, 17:5315-5322. [29] LING L, HUANG X, LI M, et al. Mapping the reactions in a single zero-valent iron nanoparticle[J]. Environmental Science & Technology, 2017, 51:14293-14300. [30] LING L,ZHANG W X. Enrichment and encapsulation of uranium with iron nanoparticle[J]. Journal of the American Chemical Society, 2015, 137:2788-2791. [31] LING L,ZHANG W X. Visualizing arsenate reactions and encapsulation in a single zero-valent iron nanoparticle[J]. Environmental Science & Technology, 2017, 51:2288-2294. [32] MARTIN J E, HERZING A A, YAN W, et al. Determination of the oxide layer thickness in core-shell zerovalent iron nanoparticles[J]. Langmuir, 2008, 24:4329-4334. [33] MU Y, JIA F, AI Z, et al. Iron oxide shell mediated environmental remediation properties of nano zero-valent iron[J]. Environmental Science:Nano, 2017, 4:27-45. [34] SUN Y P, LI X Q, CAO J, et al. Characterization of zero-valent iron nanoparticles[J]. Advances in Colloid and Interface Science, 2006, 120:47-56. [35] COLLIEX C, MANOUBI T,ORTIZ C. Electron-energy-loss-spectroscopy near-edge fine structures in the iron-oxygen system[J]. Physical Review B, 1991, 44:11402-11411. [36] STEFANIUK M, OLESZCZUK P,OK Y S. Review on nano zerovalent iron (nZVI):From synthesis to environmental applications[J]. Chemical Engineering Journal, 2016, 287:618-632. [37] GLAVEE G N, KLABUNDE K J, SORENSEN C M, et al. Chemistry of borohydride reduction of iron(Ⅱ) and iron(Ⅲ) ions in aqueous and nonaqueous media. formation of nanoscale Fe, FeB, and Fe2B powders[J]. Inorganic Chemistry, 1995, 34:28-35. [38] HAHN H. Gas phase synthesis of nanocrystalline materials[J]. Nanostructured Materials, 1997, 9:3-12. [39] CARPENTER E E, CALVIN S, STROUD R M, et al. Passivated iron as core-shell nanoparticles[J]. Chemistry of Materials, 2003, 15:3245-3246. [40] LI F, VIPULANANDAN C,MOHANTY K K. Microemulsion and solution approaches to nanoparticle iron production for degradation of trichloroethylene[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2003, 223:103-112. [41] SHAN G, YAN S, TYAGI R D, et al. Applications of nanomaterials in environmental science and engineering:Review[J]. Practice Periodical of Hazardous, Toxic, and Radioactive Waste Management, 2009, 13:110-119. [42] LI S, YAN W,ZHANG W X. Solvent-free production of nanoscale zero-valent iron (nZVI) with precision milling[J]. Green Chemistry, 2009, 11:1618-1626. [43] 杜毅, 王向宇. 新型纳米零价铁的绿色合成和改性工艺研究进展[J]. 环境化学, 2016, 35(2):337-347. DU Y, WANG X Y. Green synthesis and modification of nano zero-valent iron[J]. Environmental Chemistry, 2016, 35(2):337-347(in Chinese).
[44] HOAG G E, COLLINS J B, HOLCOMB J L, et al. Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols[J]. Journal of Materials Chemistry, 2009, 19:8671-8677. [45] NJAGI E C, HUANG H, STAFFORD L, et al. Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts[J]. Langmuir, 2011, 27:264-271. [46] WENG X, GUO M, LUO F, et al. One-step green synthesis of bimetallic Fe/Ni nanoparticles by eucalyptus leaf extract:Biomolecules identification, characterization and catalytic activity[J]. Chemical Engineering Journal, 2017, 308:904-911. [47] MACHADO S, GROSSO J P, NOUWS H P A, et al. Utilization of food industry wastes for the production of zero-valent iron nanoparticles[J]. Science of the Total Environment, 2014, 496:233-240. [48] ZHANG W X, WANG C B,LIEN H L. Treatment of chlorinated organic contaminants with nanoscale bimetallic particles[J]. Catalysis Today, 1998, 40:387-395. [49] LIEN H L,ZHANG W X. Hydrodechlorination of chlorinated ethanes by nanoscale Pd/Fe bimetallic particles[J]. Journal of Environmental Engineering, 2005, 131:4-10. [50] XU Y,ZHANG W X. Subcolloidal Fe/Ag particles for reductive dehalogenation of chlorinated benzenes[J]. Industrial & Engineering Chemistry Research, 2000, 39:2238-2244. [51] SCHRICK B, BLOUGH J L, JONES A D, et al. Hydrodechlorination of trichloroethylene to hydrocarbons using bimetallic nickel-iron nanoparticles[J]. Chemistry of Materials, 2002, 14:5140-5147. [52] BARNES R J, RIBA O, GARDNER M N, et al. Optimization of nano-scale nickel/iron particles for the reduction of high concentration chlorinated aliphatic hydrocarbon solutions[J]. Chemosphere, 2010, 79:448-454. [53] LAI B, ZHANG Y, CHEN Z, et al. Removal of p-nitrophenol (PNP) in aqueous solution by the micron-scale iron-copper (Fe/Cu) bimetallic particles[J]. Applied Catalysis B:Environmental, 2014, 144:816-830. [54] YAN W, HERZING A A, LI X Q, et al. Structural evolution of Pd-doped nanoscale zero-valent iron (nZVI) in aqueous media and implications for particle aging and reactivity[J]. Environmental Science & Technology, 2010, 44:4288-4294. [55] SUN Y P, LI X Q, ZHANG W X, et al. A method for the preparation of stable dispersion of zero-valent iron nanoparticles[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2007, 308:60-66. [56] PHENRAT T, SALEH N, SIRK K, et al. Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions[J]. Environmental Science & Technology, 2007, 41:284-290. [57] SCHRICK B, HYDUTSKY B W, BLOUGH J L, et al. Delivery vehicles for zerovalent metal nanoparticles in soil and groundwater[J]. Chemistry of Materials, 2004, 16:2187-2193. [58] ZOU Y, WANG X, KHAN A, et al. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions:A review[J]. Environmental Science & Technology, 2016, 50:7290-7304. [59] SALEH N, SARBU T, SIRK K, et al. Oil-in-water emulsions stabilized by highly charged polyelectrolyte-grafted silica nanoparticles[J]. Langmuir, 2005, 21:9873-9878. [60] SIRK K M, SALEH N B, PHENRAT T, et al. Effect of adsorbed polyelectrolytes on nanoscale zero valent iron particle attachment to soil surface models[J]. Environmental Science & Technology, 2009, 43:3803-3808. [61] JIEMVARANGKUL P, ZHANG W X,LIEN H L. Enhanced transport of polyelectrolyte stabilized nanoscale zero-valent iron (nZVI) in porous media[J]. Chemical Engineering Journal, 2011, 170:482-491. [62] HE F,ZHAO D. Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers[J]. Environmental Science & Technology, 2007, 41:6216-6221. [63] KANEL S R, GOSWAMI R R, CLEMENT T P, et al. Two dimensional transport characteristics of surface stabilized zero-valent iron nanoparticles in porous media[J]. Environmental Science & Technology, 2008, 42:896-900. [64] PHENRAT T, SALEH N, SIRK K, et al. Stabilization of aqueous nanoscale zerovalent iron dispersions by anionic polyelectrolytes:Adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation[J]. Journal of Nanoparticle Research, 2008, 10:795-814. [65] PHENRAT T, KIM H J, FAGERLUND F, et al. Particle size distribution, concentration, and magnetic attraction affect transport of polymer-modified Fe0 nanoparticles in sand columns[J]. Environmental Science & Technology, 2009, 43:5079-5085. [66] PHENRAT T, FAGERLUND F, ILLANGASEKARE T, et al. Polymer-modified Fe0 nanoparticles target entrapped NAPL in two dimensional porous media:Effect of particle concentration, NAPL saturation, and injection strategy[J]. Environmental Science & Technology, 2011, 45:6102-6109. [67] TIRAFERRI A, CHEN K L, SETHI R, et al. Reduced aggregation and sedimentation of zero-valent iron nanoparticles in the presence of guar gum[J]. Journal of Colloid and Interface Science, 2008, 324:71-79. [68] XIONG Z, ZHAO D,PAN G. Rapid and complete destruction of perchlorate in water and ion-exchange brine using stabilized zero-valent iron nanoparticles[J]. Water Research, 2007, 41:3497-3505. [69] COMBA S,SETHI R. Stabilization of highly concentrated suspensions of iron nanoparticles using shear-thinning gels of xanthan gum[J]. Water Research, 2009, 43:3717-3726. [70] QIU X, FANG Z, YAN X, et al. Emergency remediation of simulated chromium (Ⅵ)-polluted river by nanoscale zero-valent iron:Laboratory study and numerical simulation[J]. Chemical Engineering Journal, 2012, 193/194:358-365. [71] LAUMANN S, MICIĆ V,HOFMANN T. Mobility enhancement of nanoscale zero-valent iron in carbonate porous media through co-injection of polyelectrolytes[J]. Water Research, 2014, 50:70-79. [72] LIN Y H, TSENG H H, WEY M Y, et al. Characteristics of two types of stabilized nano zero-valent iron and transport in porous media[J]. Science of the Total Environment, 2010, 408:2260-2267. [73] LV D, ZHOU J, CAO Z, et al. Mechanism and influence factors of chromium(Ⅵ) removal by sulfide-modified nanoscale zerovalent iron[J]. Chemosphere, 2019, 224:306-315. [74] RAJAJAYAVEL S R C,GHOSHAL S. Enhanced reductive dechlorination of trichloroethylene by sulfidated nanoscale zerovalent iron[J]. Water Research, 2015, 78:144-153. [75] XU J, AVELLAN A, LI H, et al. Sulfur loading and speciation control the hydrophobicity, electron transfer, reactivity, and selectivity of sulfidized nanoscale zerovalent iron[J]. Advanced Materials, 2020, 32:1906910. [76] KIM E J, KIM J H, CHANG Y S, et al. Effects of metal ions on the reactivity and corrosion electrochemistry of Fe/FeS nanoparticles[J]. Environmental Science & Technology, 2014, 48:4002-4011. [77] SU Y, JASSBY D, SONG S, et al. Enhanced oxidative and adsorptive removal of diclofenac in heterogeneous fenton-like reaction with sulfide modified nanoscale zerovalent iron[J]. Environmental Science & Technology, 2018, 52:6466-6475. [78] GU Y, WANG B, HE F, et al. Mechanochemically sulfidated microscale zero valent iron:pathways, kinetics, mechanism, and efficiency of trichloroethylene dechlorination[J]. Environmental Science & Technology, 2017, 51:12653-12662. [79] QUINN J, GEIGER C, CLAUSEN C, et al. Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron[J]. Environmental Science & Technology, 2005, 39:1309-1318. [80] BERGE N D,RAMSBURG C A. Oil-in-water emulsions for encapsulated delivery of reactive iron particles[J]. Environmental Science & Technology, 2009, 43:5060-5066. [81] YAN J, HAN L, GAO W, et al. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene[J]. Bioresource Technology, 2015, 175:269-274. [82] ZHU H, JIA Y, WU X, et al. Removal of arsenic from water by supported nano zero-valent iron on activated carbon[J]. Journal of Hazardous Materials, 2009, 172:1591-1596. [83] SUN Y, DING C, CHENG W, et al. Simultaneous adsorption and reduction of U(Ⅵ) on reduced graphene oxide-supported nanoscale zerovalent iron[J]. Journal of Hazardous Materials, 2014, 280:399-408. [84] DU Q, ZHANG S, PAN B, et al. Bifunctional resin-ZVI composites for effective removal of arsenite through simultaneous adsorption and oxidation[J]. Water Research, 2013, 47:6064-6074. [85] LI A, TAI C, ZHAO Z, et al. Debromination of decabrominated diphenyl ether by resin-bound iron nanoparticles[J]. Environmental Science & Technology, 2007, 41:6841-6846. [86] ZHANG R, LI J, LIU C, et al. Reduction of nitrobenzene using nanoscale zero-valent iron confined in channels of ordered mesoporous silica[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2013, 425:108-114. [87] SUN X, YAN Y, LI J, et al. SBA-15-incorporated nanoscale zero-valent iron particles for chromium(Ⅵ) removal from groundwater:Mechanism, effect of pH, humic acid and sustained reactivity[J]. Journal of Hazardous Materials, 2014, 266:26-33. [88] HU B, CHEN G, JIN C, et al. Macroscopic and spectroscopic studies of the enhanced scavenging of Cr(Ⅵ) and Se(Ⅵ) from water by titanate nanotube anchored nanoscale zero-valent iron[J]. Journal of Hazardous Materials, 2017, 336:214-221. [89] SHENG G, ALSAEDI A, SHAMMAKH W, et al. Enhanced sequestration of selenite in water by nanoscale zero valent iron immobilization on carbon nanotubes by a combined batch, XPS and XAFS investigation[J]. Carbon, 2016, 99:123-130. [90] LIU M, WANG Y, CHEN L, et al. Mg(OH)2 supported nanoscale zero valent iron enhancing the removal of Pb(Ⅱ) from aqueous solution[J]. ACS Applied Materials & Interfaces, 2015, 7:7961-7969. [91] LIU T, YANG Y, WANG Z L, et al. Remediation of arsenic(Ⅲ) from aqueous solutions using improved nanoscale zero-valent iron on pumice[J]. Chemical Engineering Journal, 2016, 288:739-744. [92] LIU T, WANG Z L, YAN X, et al. Removal of mercury (Ⅱ) and chromium (Ⅵ) from wastewater using a new and effective composite:Pumice-supported nanoscale zero-valent iron[J]. Chemical Engineering Journal, 2014, 245:34-40. [93] ZHANG X, LIN S, CHEN Z, et al. Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution:Reactivity, characterization and mechanism[J]. Water Research, 2011, 45:3481-3488. [94] ARANCIBIA-MIRANDA N, BALTAZAR S E, GARCíA A, et al. Nanoscale zero valent supported by zeolite and montmorillonite:Template effect of the removal of lead ion from an aqueous solution[J]. Journal of Hazardous Materials, 2016, 301:371-380. [95] LI Z, WANG L, MENG J, et al. Zeolite-supported nanoscale zero-valent iron:New findings on simultaneous adsorption of Cd(Ⅱ), Pb(Ⅱ), and As(Ⅲ) in aqueous solution and soil[J]. Journal of Hazardous Materials, 2018, 344:1-11. [96] HU B, YE F, REN X, et al. X-ray absorption fine structure study of enhanced sequestration of U(Ⅵ) and Se(Ⅳ) by montmorillonite decorated with zero-valent iron nanoparticles[J]. Environmental Science:Nano, 2016, 3:1460-1472. [97] GU C, JIA H, LI H, et al. Synthesis of highly reactive subnano-sized zero-valent iron using smectite clay templates[J]. Environmental Science & Technology, 2010, 44:4258-4263. [98] YUAN N, ZHANG G, GUO S, et al. Enhanced ultrasound-assisted degradation of methyl orange and metronidazole by rectorite-supported nanoscale zero-valent iron[J]. Ultrasonics Sonochemistry, 2016, 28:62-68. [99] FU R, YANG Y, XU Z, et al. The removal of chromium (Ⅵ) and lead (Ⅱ) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI)[J]. Chemosphere, 2015, 138:726-734. [100] YUAN C,LIEN H L. Removal of arsenate from aqueous solution using nanoscale iron particles[J]. Water Quality Research Journal, 2006, 41:210-215. [101] ÇELEBI O, ÜZüM Ç, SHAHWAN T, et al. A radiotracer study of the adsorption behavior of aqueous Ba2+ ions on nanoparticles of zero-valent iron[J]. Journal of Hazardous Materials, 2007, 148:761-767. [102] ÜZüM Ç, SHAHWAN T, EROǦLU A E, et al. Application of zero-valent iron nanoparticles for the removal of aqueous Co2+ ions under various experimental conditions[J]. Chemical Engineering Journal, 2008, 144:213-220. [103] BOPARAI H K, JOSEPH M,O'CARROLL D M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles[J]. Journal of Hazardous Materials, 2011, 186:458-465. [104] HUANG P, YE Z, XIE W, et al. Rapid magnetic removal of aqueous heavy metals and their relevant mechanisms using nanoscale zero valent iron (nZVI) particles[J]. Water Research, 2013, 47:4050-4058. [105] ARANCIBIA-MIRANDA N, BALTAZAR S E, GARCíA A, et al. Lead removal by nano-scale zero valent iron:Surface analysis and pH effect[J]. Materials Research Bulletin, 2014, 59:341-348. [106] HABISH A J, LAZAREVIĆ S, JANKOVIĆ-ČASTVAN I, et al. Nanoscale zerovalent iron (nZVI) supported by natural and acid-activated sepiolites:The effect of the nZVI/support ratio on the composite properties and Cd2+ adsorption[J]. Environmental Science and Pollution Research, 2017, 24:628-643. [107] WANG C, LUO H, ZHANG Z, et al. Removal of As(Ⅲ) and As(Ⅴ) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites[J]. Journal of Hazardous Materials, 2014, 268:124-131. [108] XING M,WANG J. Nanoscaled zero valent iron/graphene composite as an efficient adsorbent for Co(Ⅱ) removal from aqueous solution[J]. Journal of Colloid and Interface Science, 2016, 474:119-128. [109] SHARMA A K, KUMAR R, MITTAL S, et al. In situ reductive regeneration of zerovalent iron nanoparticles immobilized on cellulose for atom efficient Cr(Ⅵ) adsorption[J]. RSC Advances, 2015, 5:89441-89446. [110] LI Z, DONG H, ZHANG Y, et al. Enhanced removal of Ni(Ⅱ) by nanoscale zero valent iron supported on Na-saturated bentonite[J]. Journal of Colloid and Interface Science, 2017, 497:43-49. [111] ÜZÜM Ç, SHAHWAN T, EROǦLU A E, et al. Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions[J]. Applied Clay Science, 2009, 43:172-181. [112] WANG J, LIU G, LI T, et al. Zero-valent iron nanoparticles (nZVI) supported by kaolinite for Cu(Ⅱ) and Ni(Ⅱ) ion removal by adsorption:Kinetics, thermodynamics, and mechanism[J]. Australian Journal of Chemistry, 2015, 68:1305-1315. [113] WANG Q, QIAN H, YANG Y, et al. Reduction of hexavalent chromium by carboxymethyl cellulose-stabilized zero-valent iron nanoparticles[J]. Journal of Contaminant Hydrology, 2010, 114:35-42. [114] SHI L N, ZHANG X,CHEN Z L. Removal of Chromium (Ⅵ) from wastewater using bentonite-supported nanoscale zero-valent iron[J]. Water Research, 2011, 45:886-892. [115] ZHANG Y Y, JIANG H, ZHANG Y, et al. The dispersity-dependent interaction between montmorillonite supported nZVI and Cr(Ⅵ) in aqueous solution[J]. Chemical Engineering Journal, 2013, 229:412-419. [116] PANG H, WU Y, HUANG S, et al. Macroscopic and microscopic investigation of uranium elimination by Ca-Mg-Al-layered double hydroxide supported nanoscale zero valent iron[J]. Inorganic Chemistry Frontiers, 2018, 5:2657-2665. [117] LIU H, LI M, CHEN T, et al. New synthesis of nZVI/C composites as an efficient adsorbent for the uptake of U(Ⅵ) from aqueous solutions[J]. Environmental Science & Technology, 2017, 51:9227-9234. [118] ZHOU Y, GAO B, ZIMMERMAN A R, et al. Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions[J]. Bioresource Technology, 2014, 152:538-542. [119] DONG H, DENG J, XIE Y, et al. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(Ⅵ) removal from aqueous solution[J]. Journal of Hazardous Materials, 2017, 332:79-86. [120] LI J, CHEN C, ZHANG R, et al. Nanoscale zero-valent iron particles supported on reduced graphene oxides by using a plasma technique and their application for removal of heavy-metal ions[J]. Chemistry-An Asian Journal, 2015, 10:1410-1417. [121] ZHENG Y, LIU J, LIANG J, et al. Graphitic carbon nitride materials:Controllable synthesis and applications in fuel cells and photocatalysis[J]. Energy & Environmental Science, 2012, 5:6717-6731. [122] TANG C, LING L,ZHANG W X. Pb(Ⅱ) deposition-reduction-growth onto iron nanoparticles induced by graphitic carbon nitride[J]. Chemical Engineering Journal, 2020, 387:124088. [123] BHOWMICK S, CHAKRABORTY S, MONDAL P, et al. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution:Kinetics and mechanism[J]. Chemical Engineering Journal, 2014, 243:14-23. [124] LV X, XU J, JIANG G, et al. Removal of chromium(Ⅵ) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes[J]. Chemosphere, 2011, 85:1204-1209. [125] O'CARROLL D, SLEEP B, KROL M, et al. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation[J]. Advances in Water Resources, 2013, 51:104-122. [126] LIU Y,LOWRY G V. Effect of particle age (Fe0 Content) and solution pH on nZVI reactivity:H2 evolution and TCE dechlorination[J]. Environmental Science & Technology, 2006, 40:6085-6090. [127] SHENG G, SHAO X, LI Y, et al. Enhanced removal of uranium(Ⅵ) by nanoscale zerovalent iron supported on Na-bentonite and an investigation of mechanism[J]. The Journal of Physical Chemistry A, 2014, 118:2952-2958. [128] 刘静, 刘爱荣, 张伟贤.纳米零价铁及其在环境介质中氧化后性质演变研究进展[J]. 环境化学, 2014, 33(4):576-583. LIU J, LIU A R, ZHANG W X. Review on transformation of oxidized nanoscale zero valent iron in environment media[J]. Environmental Chemistry, 2014, 33(4):576-583(in Chinese).
[129] LIU Z, ZHANG F, HOEKMAN S K, et al. Homogeneously dispersed zerovalent iron nanoparticles supported on hydrochar-derived porous carbon:simple, in situ synthesis and use for dechlorination of PCBs[J]. ACS Sustainable Chemistry & Engineering, 2016, 4:3261-3267. [130] LIU T, YANG X, WANG Z L, et al. Enhanced chitosan beads-supported Fe0-nanoparticles for removal of heavy metals from electroplating wastewater in permeable reactive barriers[J]. Water Research, 2013, 47:6691-6700. [131] SU Y, ZHANG Y, KE H, et al. Environmental remediation of chlorinated hydrocarbons using biopolymer stabilized iron loaded halloysite nanotubes[J]. ACS Sustainable Chemistry & Engineering, 2017, 5:10976-10985. [132] ZHAN J, ZHENG T, PIRINGER G, et al. Transport characteristics of nanoscale functional zerovalent iron/silica composites for in situ remediation of trichloroethylene[J]. Environmental Science & Technology, 2008, 42:8871-8876. [133] WEI A, MA J, CHEN J, et al. Enhanced nitrate removal and high selectivity towards dinitrogen for groundwater remediation using biochar-supported nano zero-valent iron[J]. Chemical Engineering Journal, 2018, 353:595-605. [134] JIA Z, SHU Y, HUANG R, et al. Enhanced reactivity of nZVI embedded into supermacroporous cryogels for highly efficient Cr(Ⅵ) and total Cr removal from aqueous solution[J]. Chemosphere, 2018, 199:232-242. [135] SHENG G, YANG P, TANG Y, et al. New insights into the primary roles of diatomite in the enhanced sequestration of UO22+ by zerovalent iron nanoparticles:An advanced approach utilizing XPS and EXAFS[J]. Applied Catalysis B-Environmental, 2016, 193:189-197. [136] LI Y, CHENG W, SHENG G, et al. Synergetic effect of a pillared bentonite support on Se(Ⅵ) removal by nanoscale zero valent iron[J]. Applied Catalysis B:Environmental, 2015, 174/175:329-335. [137] LIU Y, MAJETICH S A, TILTON R D, et al. TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties[J]. Environmental Science & Technology, 2005, 39:1338-1345. [138] BAE S, COLLINS R N, WAITE T D, et al. Advances in surface passivation of nanoscale zerovalent iron:A critical review[J]. Environmental Science & Technology, 2018, 52:12010-12025. [139] RODRIGUES R, BETELU S, COLOMBANO S, et al. Reductive dechlorination of hexachlorobutadiene by a Pd/Fe microparticle suspension in dissolved lactic acid polymers:degradation mechanism and kinetics[J]. Industrial & Engineering Chemistry Research, 2017, 56:12092-12100. [140] DE TORO J A, VASILAKAKI M, LEE S S, et al. Remanence plots as a probe of spin disorder in magnetic nanoparticles[J]. Chemistry of Materials, 2017, 29:8258-8268. [141] LING X, LI J, ZHU W, et al. Synthesis of nanoscale zero-valent iron/ordered mesoporous carbon for adsorption and synergistic reduction of nitrobenzene[J]. Chemosphere, 2012, 87:655-660. [142] HOCH L B, MACK E J, HYDUTSKY B W, et al. Carbothermal synthesis of carbon-supported nanoscale zero-valent iron particles for the remediation of hexavalent chromium[J]. Environmental Science & Technology, 2008, 42:2600-2605. [143] HE F,ZHAO D. Hydrodechlorination of trichloroethene using stabilized Fe-Pd nanoparticles:Reaction mechanism and effects of stabilizers, catalysts and reaction conditions[J]. Applied Catalysis B:Environmental, 2008, 84:533-540. -

 点击查看大图
点击查看大图
计量
- 文章访问数: 9259
- HTML全文浏览数: 9259
- PDF下载数: 396
- 施引文献: 0



 下载:
下载: