水环境中铂族元素的环境化学行为
Environmental chemical behaviors of platinum group elements in aquatic environment
-
摘要: 水环境中,由于人为来源导致铂族元素(PGE)的浓度不断增加.人们普遍认为PGE是惰性的,不会对环境造成危害,但水环境中PGE易发生化学迁移,转化为毒性更大的形态,增加生物可利用性.基于国内外79篇文献,概述了水环境PGE的环境行为,梳理了PGE的来源分布,探讨了PGE入海通量的变化和水生生物的毒性效应,分析了PGE的形态变化及影响因素.针对已有的研究成果和存在不足,对未来PGE的研究方向提出展望.Abstract: The concentrations of platinum group elements (PGE) in aquatic environment have been increasing due to human activity. It is generally assumed that anthropogenic PGE behaves in inert manner and does not pose a hazard to the environment. However, PGE might easily be mobilized and transformed into more toxic forms, enhanced their bioavailability in the aquatic environment. Based on 79 literatures, this paper summarized the research about the PGE in aquatic environment, outlined the environmental behavior of PGE, introduced the source distribution and changes of flux into the sea of PGE, discussed the toxicological response of aquatic organisms, and analysed the Speciation and influence factors of PGE. The purpose of this review was to attract more attention to the effect of PGE. In addition, in view of the existing research results and limitations, the future research directions of PGE were also outlined.
-
阻燃剂广泛应用于塑料、橡胶和纤维中,用来降低材料的可燃性[1]。2012年,中国有机磷阻燃剂产量超过17.9×104 t,在有机磷阻燃剂中,磷酸三苯酯(TPhP)是一种高产量产品,由于未与最终用途产品化学键合,故在水中可被检测到[2]。澳大利亚地表水中的TPhP浓度高达150 ng·L−1;在中国松花江,TPhP的浓度也可以达到65 ng·L−1;此外,在河南省的污水处理厂中检测到了TPhP[3-5]。在环境中出现的TPhP会通过饮食摄入和呼吸吸入,进而引起人类健康问题,有研究[6]表明,TPhP对胚胎发育、神经系统和免疫系统具有毒理作用。对于模型生物斑马鱼,当暴露于0.10 mg·L−1 TPhP时,对心脏发育具有巨大的毒性作用;通过对暴露于100 μg·L−1 TPhP的成年斑马鱼的生物蓄积和代谢研究,发现其体内TPhP浓度可高达3.12 μg·g−1[7-8]。因此,开发一种有效的材料以去除水中TPhP势在必行。
有研究[9]发现,尿素官能团化Fe3O4@LDH (urea-Fe3O4@LDH)对TPhP表现出了良好的去除效果,去除率可达90%以上,吸附容量高达589 mg·g−1,具有高效快速的吸附特性,吸附速率达到49.9 mg·(g·min)−1,且离子强度对吸附影响较小。而不同环境条件影响吸附过程中各物质的相互作用,故考察urea-Fe3O4@LDH去除TPhP受环境条件的影响情况势在必行。
天然水体中存在着悬浮颗粒物,地下水中悬浮固体的平均浓度约200 mg·L−1,在某些极端情况下,其可能接近500 mg·L−1。在这些悬浮颗粒中,大颗粒相对容易沉降,颗粒携带有机磷污染物形成沉积物,导致污染持续时间长;小颗粒容易在实际运动的水体或实验搅拌的条件下悬浮,从而与污染物更多接触,易影响污染物的去除[10-12]。水体中普遍存在的天然有机物(natural organic matter, NOM)可能改变吸附剂的表面电荷、亲疏水性和极性等性质,影响吸附剂在水体环境中的稳定性和对污染物的去除[13-14]。实际废水中一般有机和无机污染物共存,Br−作为一种无机污染物广泛存在,主要来自海水、地表水、工业废水和油田废水等,其存在极大地影响到人类的健康[15]。
实际水体中颗粒物的主要成分是黏土矿物,而富里酸(FA)、腐植酸(HA)和可溶性微生物副产物(SMPs)是NOM的最重要组分。本研究利用高岭土模拟颗粒物,用富里酸、腐植酸和牛血清白蛋白(BSA)模拟水体中存在的有机物,以及Br−作为无机污染物,考察了以上环境条件对urea-Fe3O4@LDH吸附TPhP的影响。利用urea-Fe3O4@LDH吸附剂的电荷特性和阴离子交换性,深入了解吸附过程中有机磷污染物的去除机理,为urea-Fe3O4@LDH在实际水体环境中去除有机磷污染物提供参考。
1. 材料与方法
1.1 药品及试剂
磷酸三苯酯(纯度>98%)、对甲苯基异氰酸酯和四氢呋喃(THF)购自麦克林生化科技有限公司,Fe3O4和3-氨丙基三乙氧基硅烷(APTES)购自阿拉丁生化科技股份有限公司,Na2CO3购自Sigma-Aldrich,Mg(NO3)2·6H2O、Al(NO3)3·9H2O、FA和HA购自福晨化学试剂厂,BSA购自上海金穗生物科技有限公司。
1.2 分析测试仪器
利用高效液相色谱HPLC测定溶液中的TPhP浓度,吸收波长为204 nm,流动相为甲醇溶液,体积分数为70%,流速为1.0 mL·min−1。用NOH等开发的方法测量材料的零点电荷(pHPZC)[16-17]。采用傅里叶变换红外分光光度计(FT-IR,Nicolet iS10,赛默飞世尔科技公司,美国)检测材料官能团。采用Zeta电位分析仪(ZS90,马尔文仪器有限公司,英国)测量材料电位。用紫外可见光分光光度计测量有机物在250 nm(E2)、365 nm(E3)、465 nm(E4)和665 nm(E6)处的吸光度。
1.3 吸附剂的制备
将0.35 g的Fe3O4纳米颗粒超声分散在30 mL去离子水中,与15 mL的NaOH (0.76 g)和Na2CO3 (0.64 g)混合均匀,在搅拌下,滴加15 mL的Mg(NO3)2·6H2O (2.32 g)和Al(NO3)3·9H2O (1.13 g)混合溶液(Mg2+:Al3+=3:1),搅拌20 min。然后静置,取出上清液20 mL,并加入等量乙二醇,搅拌10 min,将混合物移入110 ℃反应釜中保持12 h。将得到的固体洗涤至中性,在60 ℃下干燥,研磨得到Fe3O4@LDH粉末。将Fe3O4@LDH分散在100 mL乙醇中,加入APTES (0.1 g),在80 ℃下回流10 h,抽滤、洗涤、干燥后得到NH2-Fe3O4@LDH。将得到的材料分散到THF中,加入对甲苯基异氰酸酯0.03 g,室温下,密闭搅拌24 h,用乙醇和水洗涤,于60 ℃下干燥,得到urea-Fe3O4@LDH。
1.4 吸附剂的性能测试
取浓度为1.9 mg·L−1的TPhP溶液500 mL,加入10 mg·L−1吸附剂,以200 r·min−1搅拌6 h。配置TPhP和不同浓度的颗粒、FA、HA、BSA的混合溶液,采用0.1 mol·L−1 HCl和NaOH调节溶液pH。Br−的初始浓度为10 mg·L−1,此时吸附剂投加量为100 mg·L−1。研究在不同环境条件下urea-Fe3O4@LDH对TPhP去除率的影响,去除率和吸附量根据式(1)和式(2)进行计算。
η=(C0−Ct)C0×100% (1) qt=(C0−Ct)Vm (2) 式中:η为去除率;C0为初始溶液中TPhP的浓度,mg·L−1;Ct为时间t时TPhP的浓度,mg·L−1;t为反应时间,h;V为溶液的总体积,L;qt为时间t内单位质量吸附剂的吸附量,mg·g−1;m为吸附剂质量,g。
2. 结果与讨论
2.1 颗粒物的影响
图1为不同浓度和粒径的高岭土对urea-Fe3O4@LDH吸附TPhP的影响结果。由图1可知,在相同颗粒粒径的条件下,高岭土浓度对urea-Fe3O4@LDH去除TPhP显示出一定规律性,呈现先轻微提高后降低的变化趋势。当颗粒粒径为200~450 μm时,高岭土浓度为0~100 mg·L−1,随着浓度的增大,urea-Fe3O4@LDH对TPhP的去除率从88%提高到98%;当高岭土浓度>100 mg·L−1时,TPhP去除率略有下降,由98%降低到78%。这是由于高岭土对TPhP有一定的吸附作用(图2)。当高岭土浓度增大至100 mg·L−1时,TPhP去除率增加到37%,随着高岭土浓度提高,高岭土和吸附剂共同作用去除TPhP,因此,导致TPhP的去除率有所提高。随高岭土浓度的继续增加,TPhP的去除率降低,这是因为高岭土减少了吸附剂与TPhP的接触概率。高岭土浓度>500 mg·L−1时,TPhP去除率明显降低。
当在同一高岭土浓度下,高岭土颗粒的粒径对urea-Fe3O4@LDH吸附TPhP有一定的影响。将高岭土颗粒粒径筛分成<30、30~74、74~200和200~450 μm,随着高岭土颗粒粒径的减小,吸附剂对TPhP的去除率有所降低。这是因为TPhP分子体积较大,由于空间效应,大颗粒的高岭土更容易吸附TPhP,故导致TPhP去除率有所升高;随着高岭土颗粒粒径的减小,其对TPhP的吸附减少,另一方面,高岭土减少了urea-Fe3O4@LDH和TPhP的碰撞概率,TPhP的吸附量降低,此时吸附剂和高岭土对TPhP的去除率降低。
2.2 有机物的影响
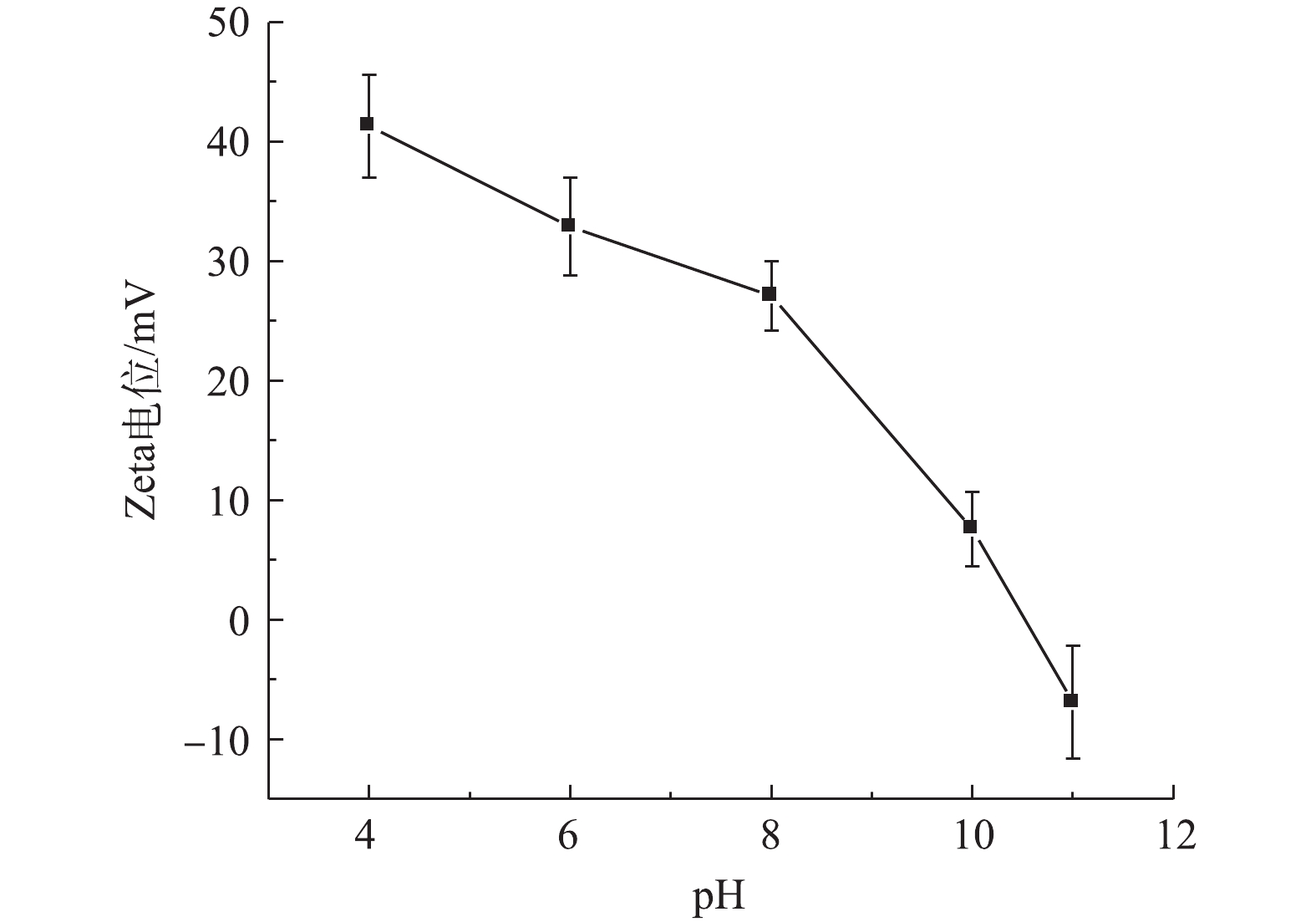
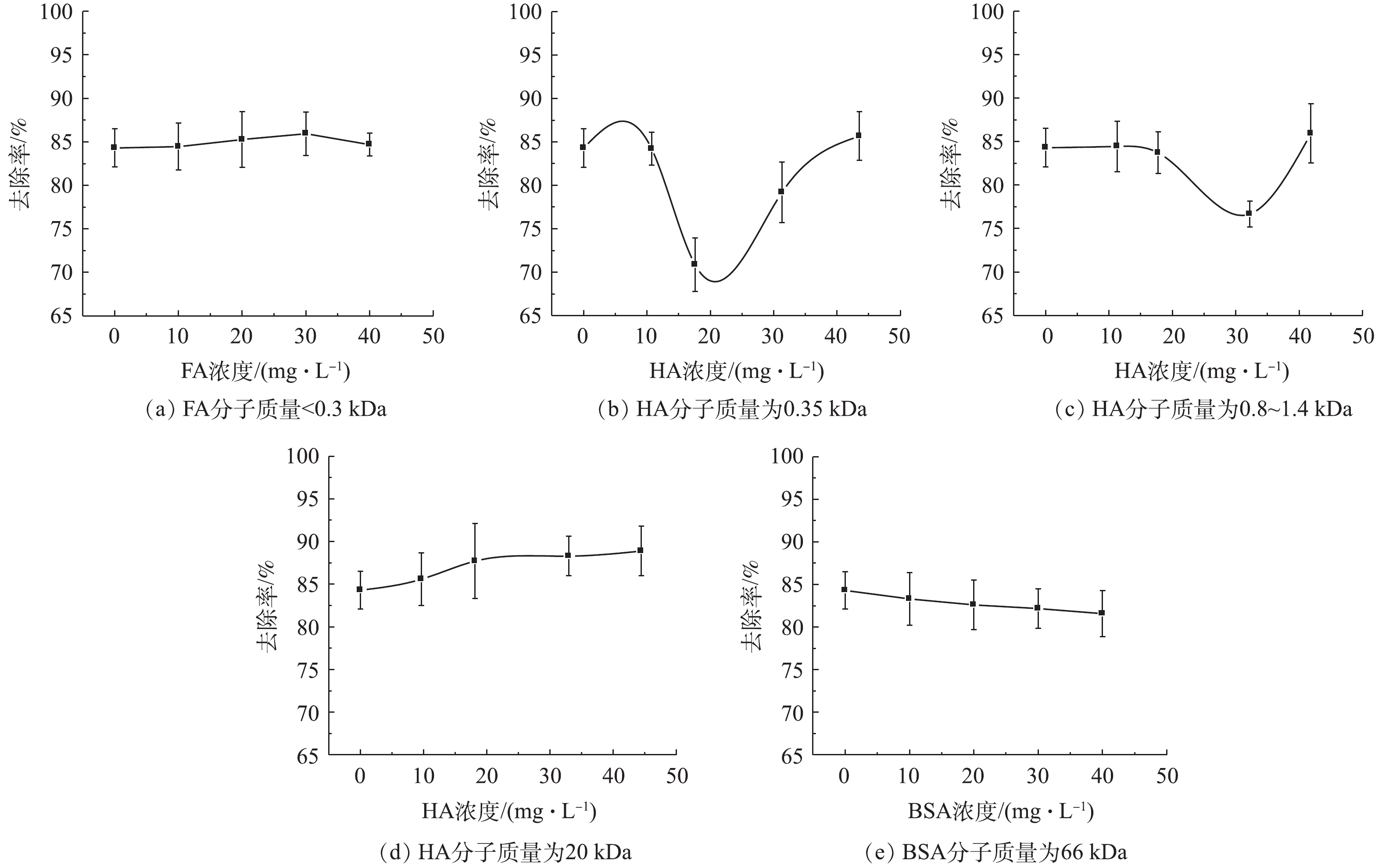
由于NOM的化学分子结构和性质不同,其与材料间的吸附和络合的相互作用均有所不同[18],因此,NOM的存在和不同分子质量有机物对urea-Fe3O4@LDH吸附TPhP具有一定的影响。图3为NOM中的FA、HA和BSA对urea-Fe3O4@LDH吸附TPhP的影响结果。如图3(a)所示,在相同pH条件下,当溶液中FA浓度由0 mg·L−1增大到200 mg·L−1时,urea-Fe3O4@LDH对TPhP的去除率降低。urea-Fe3O4@LDH吸附TPhP依靠静电相互作用,吸附剂的等电点为10.5,在pH为4~8时,其表面带有正电荷(pHpzc>pH),而FA中包含羧基、芳环、脂肪族链、和酚基等,这些酸性官能团的解离使FA带负电荷,故其可吸附到urea-Fe3O4@LDH表面,导致吸附剂表面电荷量减少,从而使得TPhP的去除率有所降低。在FA浓度相同的情况下,在pH=6时,urea-Fe3O4@LDH对TPhP的去除率高于pH=4和8时对应的去除率。由图4可知,pH在4~8时,urea-Fe3O4@LDH的Zeta电位为正,吸附剂表面带正电荷,且变化不大,因此,TPhP去除率变化主要是其基团所带电荷随pH变化所导致的。TPhP的磷酸基团中氧原子具有高的电子云密度,使得TPhP发生质子化[19],吸附剂与带正电荷的TPhP相互排斥;随着溶液pH的升高,质子化作用减弱,吸附剂表面与TPhP之间的排斥力减弱,带负电荷的氧和带正电荷的吸附剂表面之间的静电作用增强,故导致TPhP的去除率提高;继续增加溶液pH,TPhP中O=P基团带正电荷,吸附剂与TPhP中的磷酸基团发生排斥作用[20],最终导致TPhP的去除率有所降低。
图3(b)是HA对urea-Fe3O4@LDH吸附TPhP的影响结果。当pH=6和8时,随着HA浓度的增大,TPhP去除率亦随之提高。通过π-π相互作用,HA吸附在urea-Fe3O4@LDH材料表面,使吸附剂的疏水性增大,故使得疏水性较强的TPhP更易吸附在材料表面,从而有利于提高TPhP的去除率[21],此外,HA表面的芳环结构与TPhP间的π-π相互作用促进了TPhP吸附在HA上,同样可使TPhP去除率有所升高,此时HA和吸附剂均能够去除TPhP。WANG等[22]利用2种不同树脂在HA的存在下吸附有机磷阻燃剂,去除率随着HA浓度的增加基本保持恒定,其中TPhP去除率随HA浓度的增大而升高。PANG等[23]的研究发现,磁性MOF微球提取有机磷阻燃剂不受到HA的影响。然而,HA可以占据材料吸附位点,从而影响污染物的去除效果[24],因此,在pH=4时,随HA浓度升高,TPhP的去除率降低。此时质子化的TPhP带正电荷,不易吸附在带正电的urea-Fe3O4@LDH表面上,而HA中包含大量酸性位点,容易吸附在吸附材料上,占据了有效的吸附位点,随HA浓度升高,urea-Fe3O4@LDH对TPhP的去除率减少。HA对TPhP的去除率随其浓度升高趋于稳定,远低于urea-Fe3O4@LDH[9],故导致TPhP去除率降低。
图3(c)为使用BSA模拟SMPs的情况下,其对urea-Fe3O4@LDH吸附TPhP影响结果。在相同pH条件下,随着BSA浓度的增大,urea-Fe3O4@LDH对TPhP的去除率降低。这是由于BSA中含有大量氨基酸残基,可占据urea-Fe3O4@LDH表面的吸附位点;BSA带有负电荷时(pKa=4.7),容易被吸附在带正电的吸附剂表面,并且其浓度远高于TPhP,占据urea-Fe3O4@LDH的活性位点越多,TPhP的去除率越低。此外,BSA还有一定絮凝的作用,其能够促使吸附剂的沉淀,因而BSA对TPhP的去除率影响较大。TPhP的去除率在pH为6的条件下高于pH=4和8的情况下,此结果与FA、HA对TPhP去除率的影响结果一致。当溶液的pH=6和8时,BSA带负电荷,能吸附到材料表面,占据urea-Fe3O4@LDH吸附位点,导致TPhP去除率快速下降;当溶液的pH=4时,BSA表面带正电荷,占据吸附剂活性位点较少,从而使得TPhP去除率下降相对较慢。
不同有机物的表面化学性质对去除率的影响不同,为研究其对吸附剂和有机磷污染物的作用,使用紫外线吸收率E2/E3和E4/E6作为有机物芳香性和极性的指标[25]。如表1所示,NOM的芳香性和极性对比为FA>HA>BSA,FA和HA表面含有更多的苯环等芳香基团和羧基、羟基等极性基团,因此,其能够通过π-π相互作用和静电相互作用吸附到urea-Fe3O4@LDH上。在有机物浓度低于50 mg·L−1时,FA、HA能够吸附到材料表面,进而通过苯环、羧基等官能团来吸附TPhP,使得TPhP去除率较为稳定,甚至略有增大,而组成BSA的氨基酸能占据部分吸附位点,但其对TPhP吸附能力弱,造成TPhP去除率略微降低。当溶液中有机物浓度较高时,urea-Fe3O4@LDH表面吸附更多有机物,吸附剂与TPhP接触率降低,尿素官能团对TPhP吸附能力减少,TPhP去除率降低。
表 1 有机物的性质Table 1. Property of organic matters有机物 分子质量/kDa E2/E3 E4/E6 FA <0.3 4.42 15.8 HA 0.35 3.45 7.3 HA 0.8~1.4 3.36 6.9 HA 20 3.23 6.6 BSA 66 1.10 1.2 通常,FA的分子质量较小,小于0.3 kDa;HA的分子质量一般为几百到几万,以HA分子质量为3.5、0.8~1.4和20 kDa为例;BSA分子质量约为66 kDa,不同分子质量的有机物在urea-Fe3O4@LDH孔道内扩散和吸附效果有所不同。图5为有机物分子质量对urea-Fe3O4@LDH吸附TPhP的影响结果。有机物浓度为0~40 mg·L−1时,有机物分子质量对urea-Fe3O4@LDH吸附TPhP的去除率影响不大,这是由于吸附主要依靠官能团而不是吸附剂的孔道。其中,不同分子质量HA对urea-Fe3O4@LDH吸附TPhP的效率有一定影响。当HA浓度为0~40 mg·L−1时,urea-Fe3O4@LDH对TPhP去除率略有降低,当HA浓度继续增加时,对TPhP的去除率增加。HA可占据urea-Fe3O4@LDH材料表面吸附位点和填充孔隙,抑制了污染物的去除,TPhP去除率降低;同时HA能够吸附有机磷化合物,污染物疏水性越强,与HA之间的吸附系数越大,结合能力随之越强,TPhP去除率上升,因此TPhP去除率变化。
2.3 溴离子的影响
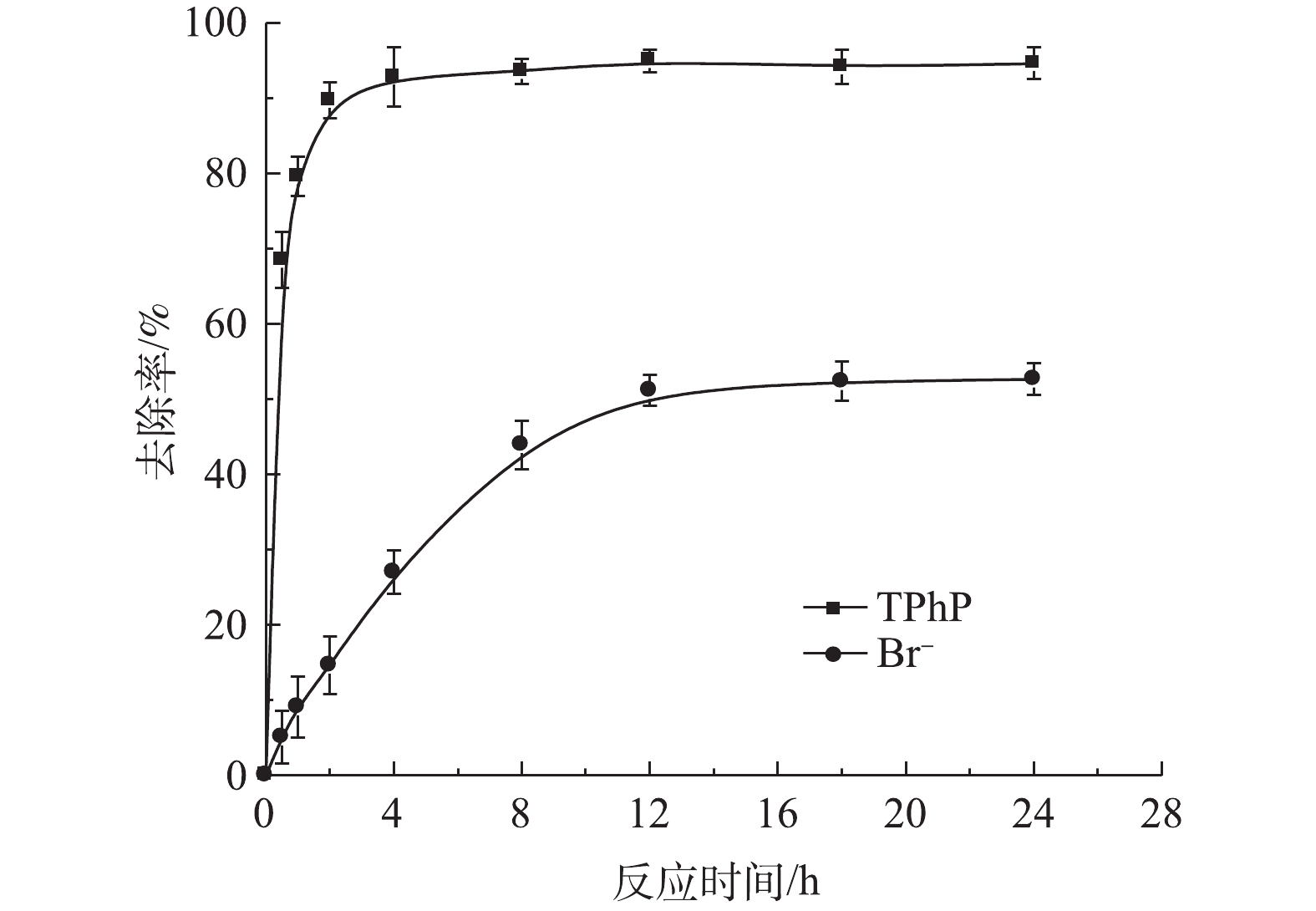
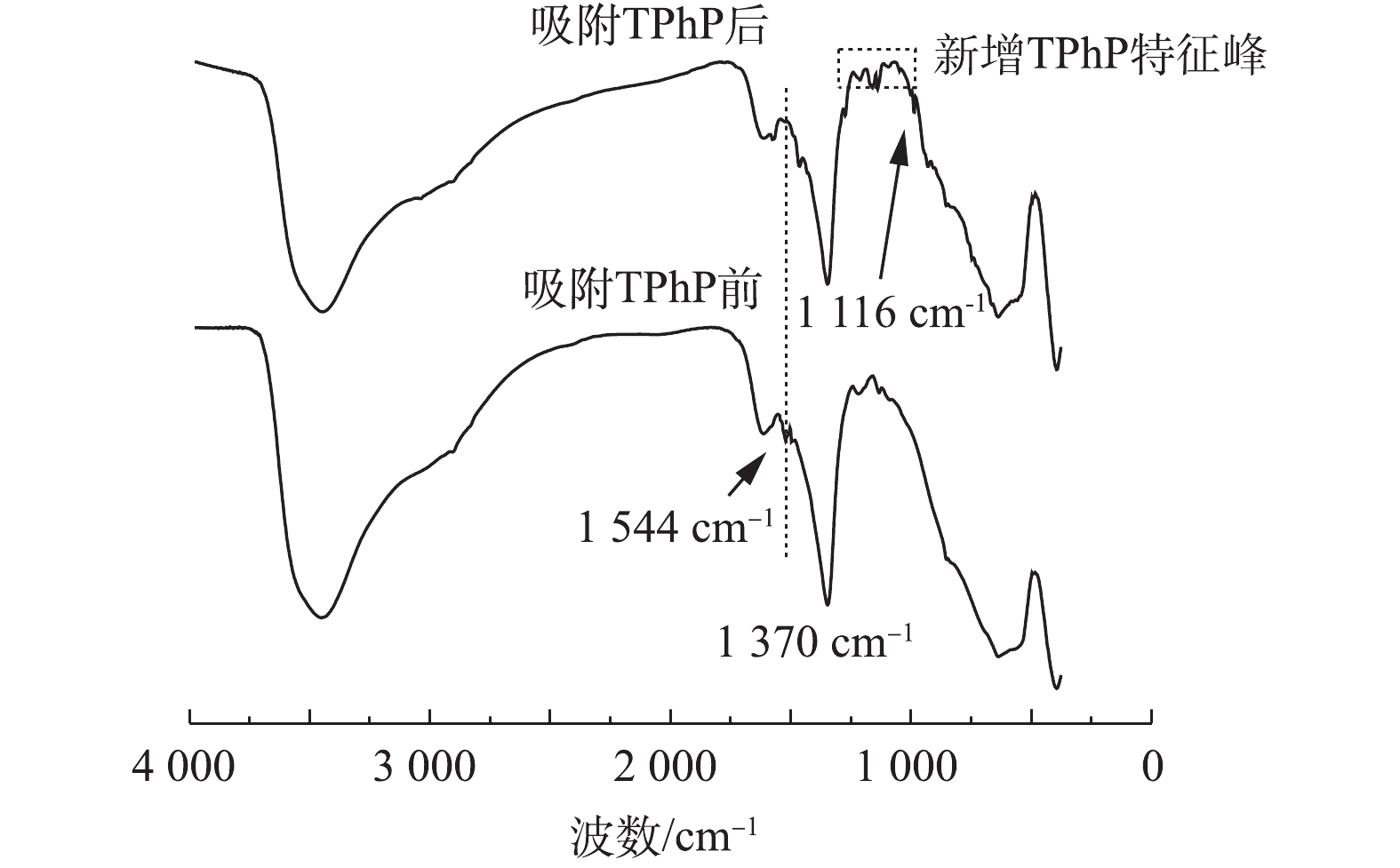
图6为在无机离子Br−存在的条件下,urea-Fe3O4@LDH对TPhP去除率的影响结果。结果表明,urea-Fe3O4@LDH对TPhP的去除率和速率并没有明显的影响,而且对Br−的去除率达到53%,因此,urea-Fe3O4@LDH可以同时吸附TPhP和无机阴离子Br−。图7为urea-Fe3O4@LDH吸附TPhP前后的FT-IR图谱。由图7可知,吸附TPhP后,材料表面出现其特征峰,在1 544 cm−1处的N—H峰有所减弱,在1 116 cm−1处的C—O峰附近出现N—O振动峰,这表明urea-Fe3O4@LDH和TPhP之间存在静电作用,在1 519 cm−1处的苯基在吸附后发生了变化,这是由于芳环间存在π-π相互作用。此外,在1 370 cm−1处的
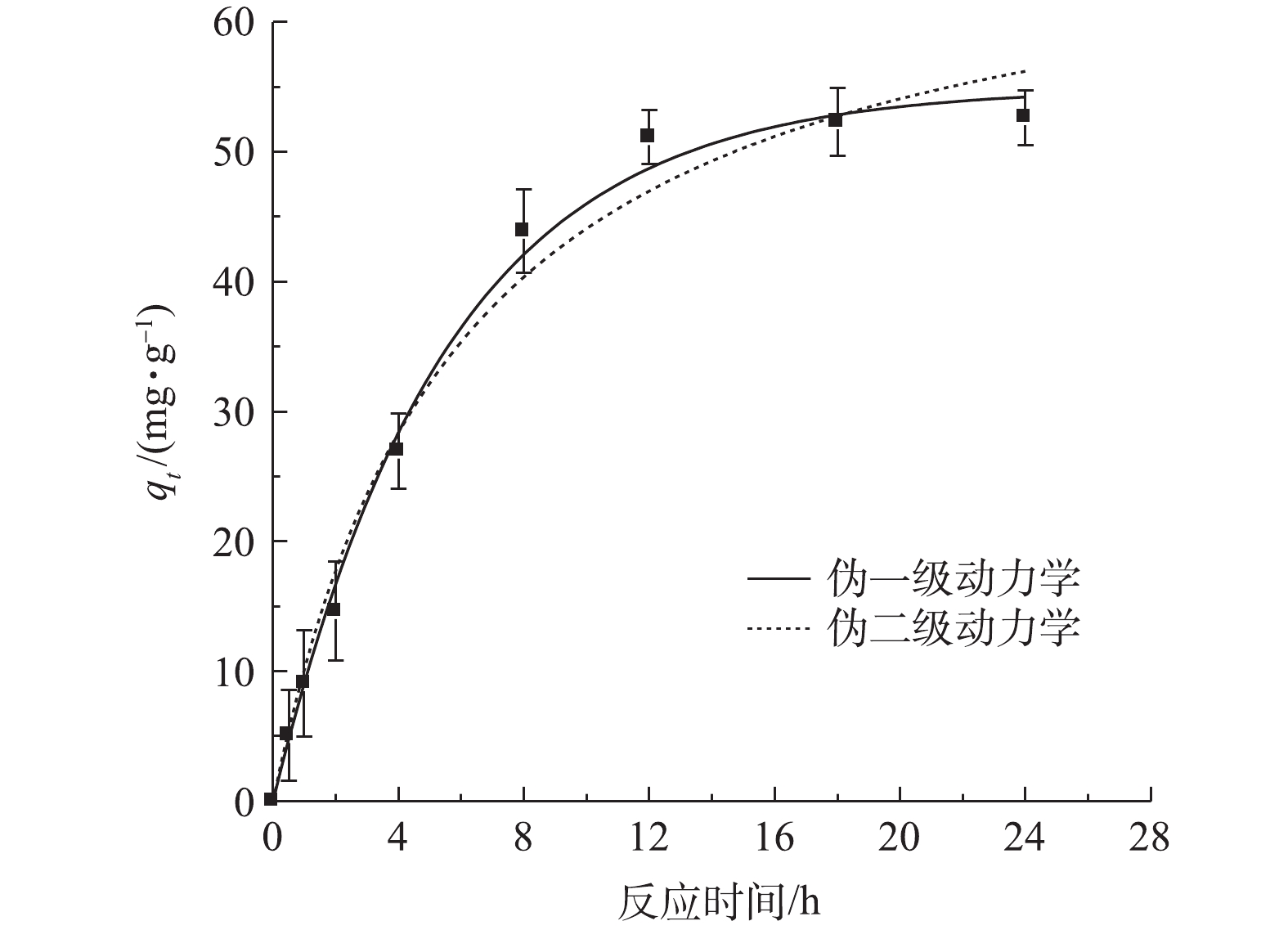
CO2−3 峰未变,表明TPhP没有进入LDH层间。加入Br−的浓度远高于TPhP,这表明TPhP和Br−在urea-Fe3O4@LDH表面没有发生竞争吸附,Br−可能通过离子交换进入层间,这是Br−吸附速率较慢的原因。urea-Fe3O4@LDH吸附Br−符合伪一级动力学方程(R2=0.995),Br−在吸附剂表面为单层吸附(图8)。通过动力学参数计算可知,Br−的最大吸附量为55 mg·g−1,吸附速率为11.9 mg·(g·h)−1。3. 结论
1)利用高岭土作为模拟颗粒物,当高岭土浓度为0~100 mg·g−1时,随着其浓度的升高,吸附剂对TPhP的去除率随之增加;当高岭土浓度>100 mg·g−1时,TPhP去除率略有下降;在同一浓度下,随着高岭土颗粒粒径的减小,TPhP去除率随之降低;高岭土对TPhP具有吸附作用,去除率最大能达到40%,且当颗粒大小为74~200 μm时,其对TPhP的吸附效果最好。
2)用FA、HA和BSA模拟水体中存在的有机物,在相同pH条件下,随着溶液中有机物浓度的增大,TPhP的去除率有所降低;在pH=6时,TPhP的去除率高于pH=4或8时对应的去除率;分子质量对TPhP去除率的影响不大。
3) urea-Fe3O4@LDH对TPhP的去除效率和速率没有受到Br−存在的影响,且能够同时吸附Br−,效率达到53%左右,吸附量达到55 mg·g−1;吸附剂去除Br−符合伪一级动力学方程,故为单层吸附。
4) urea-Fe3O4@LDH吸附主要依靠尿素官能团与TPhP间的静电作用和π-π作用;在一定浓度范围内,urea-Fe3O4@LDH去除TPhP受水体中颗粒物、有机物和无机阴离子影响较小,是一种去除废水中有机磷污染物尤其是TPhP的良好吸附剂。
-
[1] FIRMANSYAH M L, KUBOTA F, YOSHIDA W, et al. Application of a novel phosphonium-based ionic liquid to the separation of platinum group metals from automobile catalyst leach liquor[J]. Industrial & Engineering Chemistry Research, 2019, 58(9):3845-3852. [2] SUN L, WANG, B, WANG Y D. A Schottky-junction-based platinum nanoclusters@silicon carbide nanosheet as long-term stable hydrogen sensors[J]. Applied Surface Science, 2019, 473:641-648. [3] PUJA P, KUMAR P. A perspective on biogenic synthesis of platinum nanoparticles and their biomedical applications[J]. Spectrochimica Acta Part A-molecular and Biomolecular Spectroscopy, 2019, 211:94-99. [4] RINKOVEC J, PEHNEC G, GODEC R, et al. Spatial and temporal distribution of platinum, palladium and rhodium in Zagreb air[J]. Science of the Total Environment, 2018, 636:456-463. [5] LIU Y Y, FU B, SHEN Y X, et al. Seasonal properties on PM1 and PGEs (Rh, Pd, and Pt) in PM1[J]. Atmospheric Pollution Research, 2018, 9:1032-1037. [6] LIU Y Y, WANG Z C, ZHANG L, et al. Spatial and temporal distribution of platinum group elements (PGEs) in roadside soils from Shanghai and Urumqi, China[J]. Journal of Soils & Sediments, 2015, 15(9):1947-1959. [7] LEE H Y, CHON H T, SAGER M, et al. Platinum pollution in road dusts, roadside soils, and tree barks in Seoul, Korea[J]. Environmental Geochemistry & Health, 2012, 34(1):5-12. [8] KOMENDOVA R, JEZEK S. The distribution of platinum in the environment in large cities:A model study from Brno, Czech Republic[J]. International Journal of Environmental Science and Technology, 2019, 16(7):3109-3116. [9] 王娟,朱若华,施燕支.北京市区公路旁尘土中铂族元素的化学形态[J]. 环境化学,2007,26(4):528-530. WANG J, ZHU R H, SHI Y Z. Speciation of platinum group elements in dust near highway in Beijing[J]. Environmental Chemistry, 2007, 26(4):528-530(in Chinese).
[10] SOYOLERDENE T O, HUH Y, HONG S, et al. A 50-year record of platinum, iridium, and rhodium in Antarctic snow:Volcanic and anthropogenic sources[J]. Environmental Science & Technology, 2011, 45(14):5929-5935. [11] ESSUMANG D K. Bioaccumulation of platinum group metals in dolphins, Stenella sp. caught off Ghana[J]. Journal of the Limnological Society of Southern Africa, 2008, 33(3):255-259. [12] TURNER A, PEDROSO S S, BROWN M T. Influence of salinity and humic substances on the uptake of trace metals by the marine macroalga, Ulva lactuca:experimental observations and modelling using WHAM. Mar Chem[J]. Marine Chemistry, 2008, 110(3):176-184. [13] DUBIELLAJACKOWSKA A, KUDAK B, POLKOWSKA Z, et al. Environmental fate of traffic-derived platinum group metals[J].Critical Reviews in Analytical Chemistry, 2009, 39:251-271. [14] SEN IS, PEUCKER-EHRENBRINK B. Anthropogenic disturbance of element cycles at the Earth's surface[J]. Environmental Science & Technology, 2012, 46:8601-8609. [15] PAN S H, ZHANG G, SUN Y L, et al. Accumulating characteristics of platinum group elements (PGE) in urban environments, China[J]. Science of the Total Environment, 2009, 407(14):4248-4252. [16] ESSUMANG D K, DODOO D K, ADOKOH C K, et al. Bioaccumulation of platinum group metals on some fish species (and) in the Pra estuary of Ghana[J]. Toxicological & Environmental Chemistry Reviews, 2008, 90(3):625-638. [17] MULHOLLAND R, TURNER A. Accumulation of platinum group elements by the marine gastropod Littorina littorea[J]. Environmental Pollution, 2011, 159(4):977-982. [18] ABDEL-TAWWABA M, RAZEKB N A, ABDEL-RAHMANB A M. Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.)[J]. Fish and Shellfish Immunology, 2019, 88:254-258. [19] AUGUSTEA M, BALBIA T, MONTAGNAA M, et al. In vivo immunomodulatory and antioxidant properties of nanoceria (nCeO2) in the marine mussel Mytilus galloprovincialis[J]. Comparative Biochemistry and Physiology, Part C, 2019, 219:96-102. [20] 王金坑.入海污染物总量控制技术与方法[M]. 北京:海洋出版社,2013. WANG J K. Control technology and method of total amount of pollutants entering the sea[M]. Beijing:China Ocean Press, 2013(in Chinese). [21] 兰青,陈英.城市环境铂族金属分布特性及生态健康风险研究进展[J]. 生态环境学报,2013,22(5):894-900. LAN Q, CHEN Y. Research progress on the distribution characteristics and ecological health risk of platinum-group metal[J]. Ecology and Environmental Sciences, 2013, 22(5):894-900(in Chinese).
[22] 李培苗,高学鲁.环境中人为来源的铂族元素及其迁移转化研究进展[J]. 应用生态学报,2012,23(12):514-525. LI P M,GAO X L. Migration and transformation of anthropogenic platinum group elements in environment:Areview[J]. Chinese Journal of Applied Ecology, 2012, 23(12):514-525(in Chinese).
[23] SPADA N, BOZLAKER A, CHELLAM S. Multi-elemental characterization of tunnel and road dusts in Houston, Texas using dynamic reaction cell-quadrupole-inductively coupled plasma-mass spectrometry:Evidence for the release of platinum group and anthropogenic metals from motor vehicles[J]. Analytica Chimica Acta, 2012, 735(14):1-8. [24] 高博,陆瑾,周怀东,等.北京市高校内道路尘土中铂族元素(PGEs)污染特征[J]. 环境化学,2011,30(6):1204-1205. GAO B, LU J, ZHOU H D. Pollution characteristics of platinum group elements (PGEs) in road dust of universities in Beijing[J]. Environmental Chemistry, 2011,30(6):1204-1205(in Chinese).
[25] LINDE S J L, FRANKEN A, PLESSIS J L D. Urinary excretion of platinum from South African precious metals refinery workers[J]. Occupational & Environmental Medicine, 2018, 75(6):422-436. [26] DUBIELLAJACKOWSKA A, POLKOWSKA, NAMIENŃIK J. Platinum group elements in the environment:Emissions and exposure[J]. Reviews of Environmental Contamination and Toxicology, 2008, 199:111-135. [27] NAVARRO E, BAUN A, BEHRA R, et al. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi[J]. Ecotoxicology, 2008, 17:372-386. [28] KAREL F, MORTIER S T F. C., JANIS B, et al. Modelling and sensitivity analysis of urinary platinum excretion in anticancer chemotherapy for the recovery of platinum[J]. Sustainable Chemistry and Pharmacy, 2016, 4:46-56. [29] LENZ K, HANN S, KOELLENSPERGER G, et al. Presence of cancerostatic platinum compounds in hospital wastewater and possible elimination by adsorption activated sludge[J]. Science of the Total Environment, 2005, 345:141-152. [30] MASHIO A S, OBATA H, TAZOE H, et al. Dissolved platinum in rainwater, river water and seawater around Tokyo Bay and Otsuchi Bay in Japan[J]. Estuarine Coastal & Shelf Science, 2016, 180:160-167. [31] LIU Y Y, TIAN F F, LIU C, et al. Platinum group elements in the precipitation of the dry region of Xinjiang and factors affecting their deposition to land:The case of Changii City, China[J]. Atmospheric Pollution Research, 2015, 6(2):178-183. [32] COBELO-GARCÍA A, LÓPEZ-SÁNCHEZ D E, ALMÉCIJA C, et al. Behavior of platinum during estuarine mixing (Pontevedra Ria, NW Iberian Peninsula)[J]. Marine Chemistry, 2013, 150(2):11-18. [33] COBELO-GARCÍA A, LÓPEZ-SÁNCHEZ D E, SCHÄFER J, et al. Behavior and fluxes of Pt in the macrotidal Gironde Estuary (SW France)[J]. Marine Chemistry, 2014, 167:93-101. [34] COBELO-GARCÍA A, NEIRA P, MIL-HOMENS M, et al. Evaluation of the contamination of platinum in estuarine and coastal sediments (Tagus Estuary and Prodelta, Portugal)[J]. Marine Pollution Bulletin, 2011, 62(3):646-650. [35] OBATA H, YOSHIDA T, OGAWA H. Determination of picomolar levels of platinum in estuarine waters:A comparison of cathodic stripping voltammetry and isotope dilution-inductively coupled plasma mass spectrometry[J]. Analytica Chimica Acta, 2006, 580(1):32-38. [36] PRATT C, LOTTERMOSER B G. Mobilisation of traffic-derived trace metals from road corridors into coastal stream and estuarine sediments, Cairns, northern Australia[J]. Environmental Geology (Berlin), 2007, 52(3):437-448. [37] PRICHARD H M, JACKSON M T, SAMPSON J. Dispersal and accumulation of Pt, Pd and Rh derived from a roundabout in Sheffield (UK):From stream to tidal estuary[J]. Science of the Total Environment, 2008, 401(1):90-99. [38] GHAFURIA Y, YUNESIAN M, NABIZADEH R, et al. Environmental risk assessment of platinum cytotoxic drugs:A focus on toxicity characterization of hospital effluents[J]. International Journal of Environmental Science and Technology, 2018, 15(9):1983-1990. [39] VYAS N, TURNER A, SEWELL G. Platinum-based anticancer drugs in waste waters of a major UK hospital and predicted concentrations in recipient surface waters[J]. Science of the Total Environment, 2014, 493:324-329. [40] ZHAO M C, WADA N, SHINOZAKI H, et al. Monitoring of the palladium concentration in river water and sediment at an acidic hot spring spa area in gunma prefecture[J]. Analytical Sciences, 2018, 34(12):1357-1364. [41] LÓPEZ-SÁNCHEZA D E, COBELO-GARCÍA A, RIJKENBERG M J A, et al. Newin sights on the dissolved platinum behavior in the Atlantic Ocean[J]. Chemical Geology, 2019, 511:204-211. [42] SUZUKI A, OBATA H, OKUBO A, et al. Precise determination of dissolved platinum in seawater of the Japan Sea, Sea of Okhotsk and western North Pacific Ocean[J]. Marine Chemistry, 2014, 166:114-121. [43] WEDEPOHL K H. The composition of the continental-crust[J]. Geochimica Et Cosmochimica Acta, 1995, 59:1217-1232. [44] SOYOL-ERDENE T O, HUH Y. Dissolved platinum in major rivers of East Asia:Implications for the oceanic budget[J]. Geochemistry Geophysics Geosystems, 2012, 13(6):1-3. [45] BENCS L, RAVINDRA K, GRIEKEN R V. Methods for the determination of platinum group elements originating from the abrasion of automotive catalytic converters[J]. Spectrochim Acta Part B, 2003, 58:1723-1755. [46] DIEHL D B, GAGNON Z E. Interactions between essential nutrients with platinum group metals in submerged aquatic and emergent plants[J]. Water Air Soil Pollution, 2007, 184:255-267. [47] VANNINI C, DOMINGO G, MARSONI M, et al. Physiological and molecular effects associated with palladium treatment in Pseudokirchneriella subcapitata[J]. Aquatic Toxicology, 2011, 102:104-113. [48] SØRENSEN S N, ENGELBREKT C, LUTZHOFT H C, et al. A multimethod approach for investigating algal toxicity of platinum nanoparticles[J]. Environmental Science & Technology, 2016, 50:10635-10643. [49] MONTEIRO C M, CASTRO P M L, MALCATA F X. Metal uptake by microalgae:Underlying mechanisms and practical applications[J]. Biotechnology Progress, 2012, 28(2):299-311. [50] ZEREINI F, WISEMAN C L S. Platinum metals in the environment[M]. Berlin:Springer -Verlag Berlin Heidelberg, 2015. [51] ZIMMERMANN S, ALT F, MESSERSCHMIDT J, et al. Biological availability of traffic-related platinum-group elements (palladium, platinum, and rhodium) and other metals to the zebra mussel (Dreissena polymorpha) in water containing road dust[J]. Environmental Toxicology and Chemistry, 2002, 21:2713-2718. [52] SURES B, THIELEN F, BASKA F, et al. The intestinal parasite Pomphorhynchus laevis as a sensitive accumulation indicator for the platinum group metals Pt, Pd, and Rh[J]. Environmental Research, 2005, 98:83-88. [53] MOLDOVAN M, RAUCH S, GOMEZ M, et al. Bioaccumulation of palladium, platinum and rhodium from urban particulates and sediments by the freshwater isopod Asellus aquaticus[J]. Water Research, 2001, 35:4175-4183. [54] ESSUMANG D K, ADOKOH C K, BOAMPONSEM L. Levels of platinum group metals in selected species (Sarotherodon melanotheron, Chonophorus lateristriga, Macrobrachium vollenhovenii and Crassostrea tulipa) in some estuaries and lagoons along the coast of Ghana[J]. Scientific World Journal, 2010, 10(1):1971-1987. [55] ZIMMERMANN S, MESSERSCHMIDT J, VON B A, et al. Uptake and bioaccumulation of platinum group metals (Pd, Pt, Rh) from automobile catalytic converter materials by the zebra mussel (Dreissena polymorpha)[J]. Environmental Research, 2005, 98(2):203-209. [56] SURES B, ZIMMERMANN S. Impact of humic substances on the aqueous solubility, uptake and bioaccumulation of platinum, palladium and rhodium in exposure studies with Dreissena polymorpha[J]. Environmental Pollution, 2007, 146:444-451. [57] LI L, HOU Y, YU J, et al. Synergism of ursolic acid and cisplatin promotes apoptosis and enhances growth inhibition of cervical cancer cells via suppressing NF-κB p65.[J]. Oncotarget, 2017, 8(57):97416-97427. [58] RAVINDRA K, BENCS L, GRIEKEN R V. Platinum group elements in the environment and their health risk[J]. Science of the Total Environment, 2004, 318:1-43. [59] ASHARANI P V, LIANWU Y, GONG Z, et al. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos[J]. Nanotoxicology, 2011, 5(1):43-54. [60] CEDERVALL T, LYNCH I, LINDMAN S, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles[J]. Proceedings of the National Academy of Sciences, 2007, 104(7):2050-2055. [61] ASHARANI P V, MUN G L K, HANDE M P, et al. Cytotoxicity and genotoxicity of silver nanoparticles in human cells[J]. Acs Nano, 2009, 3(2):279-290. [62] DOHNALOVÁ L, DOHNAL V. Nanoparticles and their toxicity[J]. Chemicke Listy, 2015, 109:444-450. [63] HLAVKOVA D, BEKLOVA M, KOPEL P, et al. Evaluation of platinum nanoparticles ecotoxicity using representatives of distinct trophic levels of aquatic biocenosis[J]. Neuro Endocrinology Letters, 2019, 39(6):465-472. [64] LIU K, GAO X L, LI L. Determination of ultra-trace Pt, Pd and Rh in seawater using an off-line pre-concentration method and inductively coupled plasma mass spectrometry[J]. Chemosphere, 2018:212:429-437. [65] 刘凯,高学鲁,李力.海水中痕量铂族元素的赋存形态及螯合树脂富集研究进展[J]. 应用生态学报,2017,28(10):3424-3432. LIU K, GAO X L, LI L. Advances in trace platinum group elements speciation and preconcentration of chelate resins in seawater[J]. Chinese Journal of Applied Ecology, 2017, 28(10):3424-3432(in Chinese).
[66] TURNER A. Particle-water interactions of platinum group elements under estuarine conditions[J]. Marine Chemistry, 2007, 103(1/2):103-111. [67] COBELO-GARCÍA A, TURNER A, MILLWARD G E. Fractionation and reactivity of platinum group elements during estuarine mixing[J]. Environmental Science & Technology, 2008, 42(4):1096-1101. [68] COSDEN J M, BYRNE R H. Comparative geochemistries of Pd II, and Pt II:Formation of mixed hydroxychloro and chlorocarbonato-complexes in seawater[J]. Geochimica Et Cosmochimica Acta, 2003, 67(7):1331-1338. [69] COLOMBO C, OATES C J, MONHEMIUS A J, et al. Complexation of platinum, palladium and rhodium with inorganic ligands in the environment[J]. Geochemistry:Exploration, Environment, Analysis, 2008, 8:1-11. [70] SHAMS L, TURNER A, MILLWARD G E, et al. Extra- and intra-cellular accumulation of platinum group elements by the marine microalga, Chlorella stigmatophora[J]. Water Research, 2014, 50(1):432-440. [71] TURNER A, XU J. Influence of ionic surfactants on the flocculation and sorption of palladium and mercury in the aquatic environment[J]. Water Research, 2008, 42(1):318-326. [72] TURNER A, CRUSSELL M, MILLWARD G E, et al. Adsorption kinetics of platinum group elements in river water[J]. Environmental Science & Technology, 2006, 40(5):1524-1531. [73] 张莉,史玲珑.洱海悬浮颗粒物时空分布特征及其环境学意义[J]. 环境科学研究,2019,32(5):787-794. ZHANG L, SHI L L. Spatial and temporal distribution of suspended particulate matter in lake erhai and its environmental significance[J]. Researsh of Environmental Sciences, 2019, 32(5):787-794(in Chinese).
[74] REITH F, ZAMMIT C M, SHAR S S, et al. Biological role in the transformation of platinum-group mineral grains[J]. Nature Geoscience, 2016, 9(4):294-298. [75] TURNER A, MILLWARD G E. Suspended particles:Their role in estuarine biogeochemical cycles[J]. Estuarine Coastal and Shelf Science, 2002, 55(6):857-883. [76] TURNER A, WU K T. Removal of platinum group elements in an estuarine turbidity maximum[J]. Marine Chemistry, 2007, 107:295-307. [77] KRAEMER S M. Iron oxide dissolution and solubility in the presence of siderophores[J]. Aquatic Sciences, 2004, 66:3-18. [78] DAHLHEIMER S R, NEAL C R, FEIN J B. Potential mobilization of platinum-group elements by siderophores in surface environments[J]. Environmental Science&Technology, 2007, 41:870-875. [79] LUSTIG S, ZANG S, MICHALKE B, SCHRAMEL P, et al. Transformation behaviour of different platinum compounds in a clay-like humic soil:Speciation investigations[J]. Science of the Total Environment, 1996, 188:195-204. -

 点击查看大图
点击查看大图
计量
- 文章访问数: 4239
- HTML全文浏览数: 4239
- PDF下载数: 88
- 施引文献: 0




 下载:
下载: