畜禽养殖业主要废弃物处理工艺消除抗生素研究进展
Removal of antibiotics in waste and wastewater treatment facilities of animal breeding industry: A review
-
摘要: 随着集约化畜牧养殖业的不断发展,抗生素被广泛用作饲料添加剂以控制畜禽疾病和促进畜禽生长.用于畜禽养殖业中的抗生素绝大部分都不能被动物体所完全吸收,而是以原形或代谢物的形式随动物的粪便排出体外,然后通过各种途径进入到土壤和水体中,给人类健康带来巨大威胁.本文综述了好氧堆肥、厌氧发酵、高级氧化和人工湿地对畜禽粪便和养殖废水中抗生素的去除效果,重点讨论了不同运行参数对处理工艺去除抗生素的影响,最后根据现行研究仍存在的问题提出今后的工作建议,为畜禽养殖业抗生素污染的控制提供参考.Abstract: With the development of intensive animal husbandry, antibiotics are widely used as feed additives to control livestock disease and promote animal growth. However, instead of being assimilated by animal guts, high percentage of antibiotics are excreted out as prototype or metabolites with animal feces and enter the water and soil through various pathways, thus posing potential risks to human health. This article summarizes the performance of aerobic compost, anaerobic fermentation, advanced oxidation and constructed wetlands on the removal of antibiotics in livestock manure and wastewater. We focus on discussing the effects of different operation parameters on treatment processes for antibiotics removal. Finally, according to existing problems, this review puts forward some future research proposals for the control of antibiotic pollution in livestock and poultry breeding.
-
Key words:
- antibiotics /
- aerobic composting /
- anaerobic digestion /
- advanced oxidation /
- constructed wetlands
-
反硝化除磷菌(DPBs)具有与普通聚磷菌类似的代谢机理。在厌氧条件下DPBs利用胞内聚磷(Poly-P)及糖原(Gly)分解所获得的能量将挥发性脂肪酸(VFA)转移至体内合成PHA,宏观表现为
PO3−4 -P浓度的升高,该阶段为释磷阶段。在缺氧条件下,DPBs利用NO−x -N代替O2作为电子受体,将厌氧合成的PHA分解,产生的能量用于吸收PO3−4 -P并合成Poly-P存储于胞内,同时伴随着糖原的再生,即缺氧吸磷,从而实现了氮磷的同步去除[1]。目前大多数的反硝化除磷研究都是以NO−3 -N作为电子受体,并且效果良好。而NO−2 -N是硝化和反硝化过程的中间产物,DPBs若能以其作为电子受体则能减少碳源消耗及曝气量,并且由于其生长速率相对较慢,因此,也能减少污泥的产量。近期研究[2-3]表明,当NO−2 -N浓度较低时,DPBs能够以NO−2 -N为电子受体吸磷,并且未受到抑制。而短程硝化与全程硝化相比具有节省曝气量、反应速率快等优点[4],因此,短程反硝化除磷工艺受到更多学者的关注。近年来,多数研究者采用批次实验验证了
NO−2 -N作为反硝化除磷电子受体的可行性,然而,鲜有研究者考察NO−2 -N对长期运行效能的影响。ABR反应器具有微生物相分离以及对底物不同阶段和程度转化的优势,可产生VFA等优质碳源,同时,MBR具有高效的生物截留作用而被日益广泛地使用。本研究采用ABR-MBR工艺,驯化DPBs对NO−2 -N的耐受程度,考察了在长期运行条件下NO−2 -N对反硝化吸磷的抑制程度及耐受限度,以期寻找出最佳的运行负荷,以实现碳氮磷的同步高效去除。1. 材料与方法
1.1 实验装置及运行
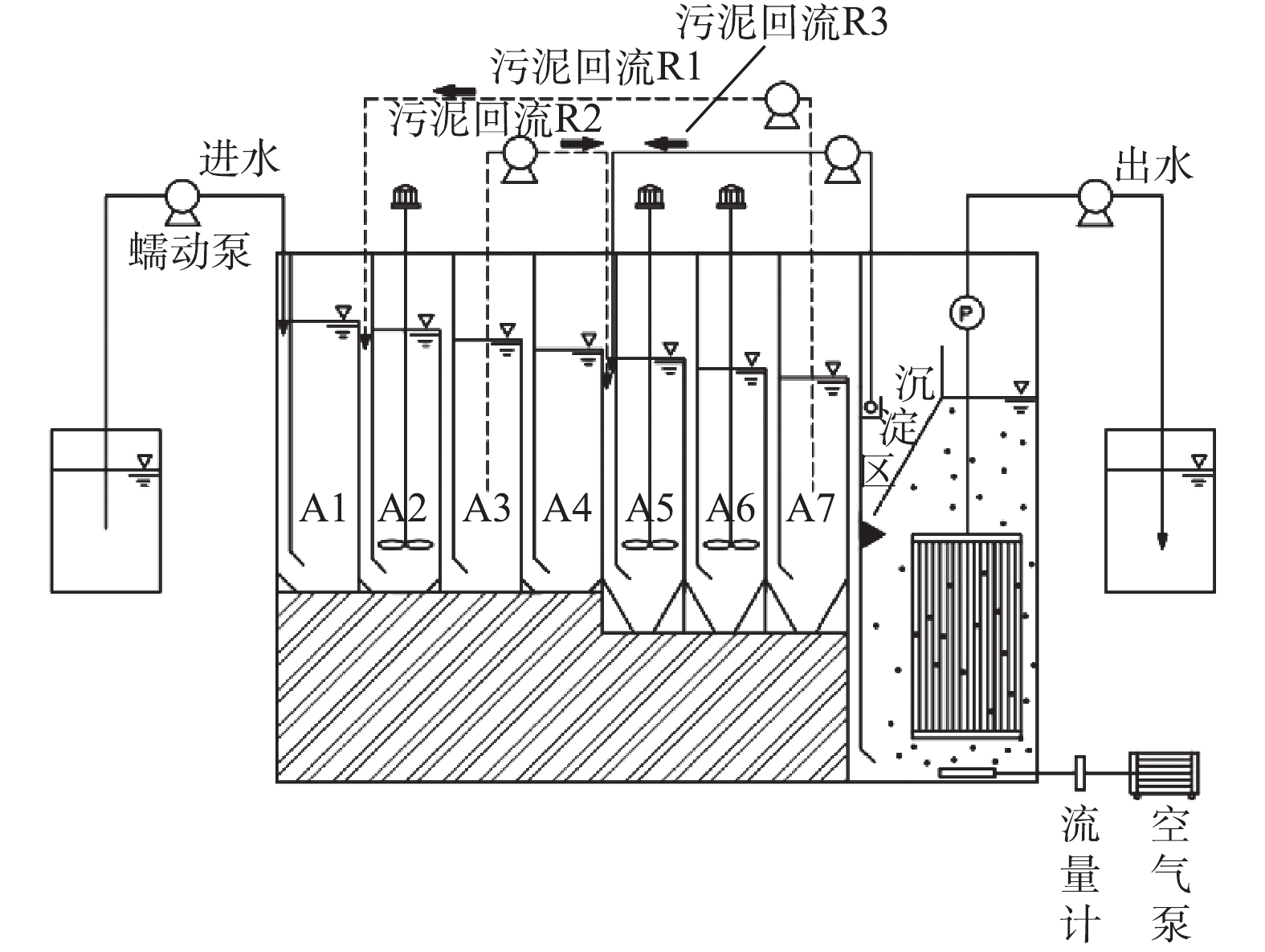
ABR-MBR工艺实验装置及处理流程如图1所示,其由含有7个隔室的ABR反应器及好氧MBR反应器组成。反应器总有效容积为11.4 L,其中ABR有效容积为7.6 L,A1~A4为厌氧区,A5~A7为缺氧区,MBR设有沉淀区进行硝化液回流,有效容积为3.8 L,采用间歇抽吸出水,抽吸周期为10 min(8 min抽吸出水和2 min反冲洗)。ABR-MBR工艺设有3个回流为R1、R2、R3。污泥回流R1(污泥为A7~A2),将富含DPBs的污泥回流至厌氧A2隔室,通过A1隔室的水解酸化作用,旨在为DPBs提供优质碳源;污泥回流R2(污泥为A3~A5),利用A4隔室对剩余碳源进一步降解,消除传统反硝化菌对DPBs电子受体的竞争;硝化液回流R3从MBR沉淀区回流至A5,为DPBs提供电子受体。
表1为实验过程及其中对应的参数。由表1可知,实验分为4个工况,第1工况共运行22 d,其他每个工况均运行14 d。实验前152 d主要包括反硝化除磷的启动和考察HRT对系统的影响,得出最佳条件:ABR反应器HRT为9 h、污泥回流比保持在80%、硝化液回流比稳定在300%、反硝化除磷功能区(A2、A3、A5~A7)污泥龄(SRT)为25 d。控制MBR反应器内溶解氧在0.5~1.0 mg·L−1,水温通过水浴加热维持在(30±2) ℃,污泥龄为15 d。控制系统进水C/N/P值不变,逐步提高进水基质浓度,稳定MBR内短程硝化的运行,以实现ABR-MBR短程反硝化除磷工艺的优化与稳定。
表 1 实验过程及参数Table 1. Experimental process and parameters工况 HRT/h COD/(mg·L−1) TN/(mg·L−1) TP/(mg·L−1) VLR/(kg·(m3·d)−1) ABR MBR A(153~174 d) 9 4.5 380 60 7.5 1.02 B(175~190 d) 9 4.5 570 80 11 1.53 C(191~204 d) 9 4.5 760 120 15 2.04 D(205~218 d) 9 4.5 950 150 18 2.55 1.2 分析方法
COD、
NH+4 -N、NO−2 -N、NO−3 -N、PO3−4 -P、TN等指标采用标准方法[5]测定,水样采用0.45 μm中速滤纸过滤,以去除悬浮物的影响,其中COD采用快速消解法;NH+4 -N采用纳氏试剂光度法测定;NO−2 -N采用N-(1-萘基)-乙二胺光度法测定;NO−3 -N采用紫外分光光度法;PO3−4 -P采用钼锑抗分光光度法;TN采用过硫酸钾氧化-紫外分光光度法;MLSS采用滤纸称重法测定。亚硝酸盐积累率(NAR)按照式(1)计算。游离亚硝酸(FNA)及游离氨(FA)值按照式(2)和式(3)进行计算。
η=CNO−2−NCNO−2−N+CNO−3−N×100% (1) 式中:η为亚硝酸盐积累率,%;
CNO−2−N 和CNO−3−N 为反应器中NO−2 -N及NO−3 -N的质量浓度,mg·L−1。CFNA=CNO−2−Ne−2300/(273+T)×10pH (2) CFA=1714×CNH4+−N×10pHe6344/(273+T)+10pH (3) 式中:CFNA为游离亚硝酸的浓度,mg·L−1;CFA为游离氨的浓度,mg·L−1;T为该系统的温度,℃;
CNH4+−N 为反应器中NH+4 -N的质量浓度,mg·L−1。2. 结果与讨论
2.1 基质浓度对短程硝化的影响
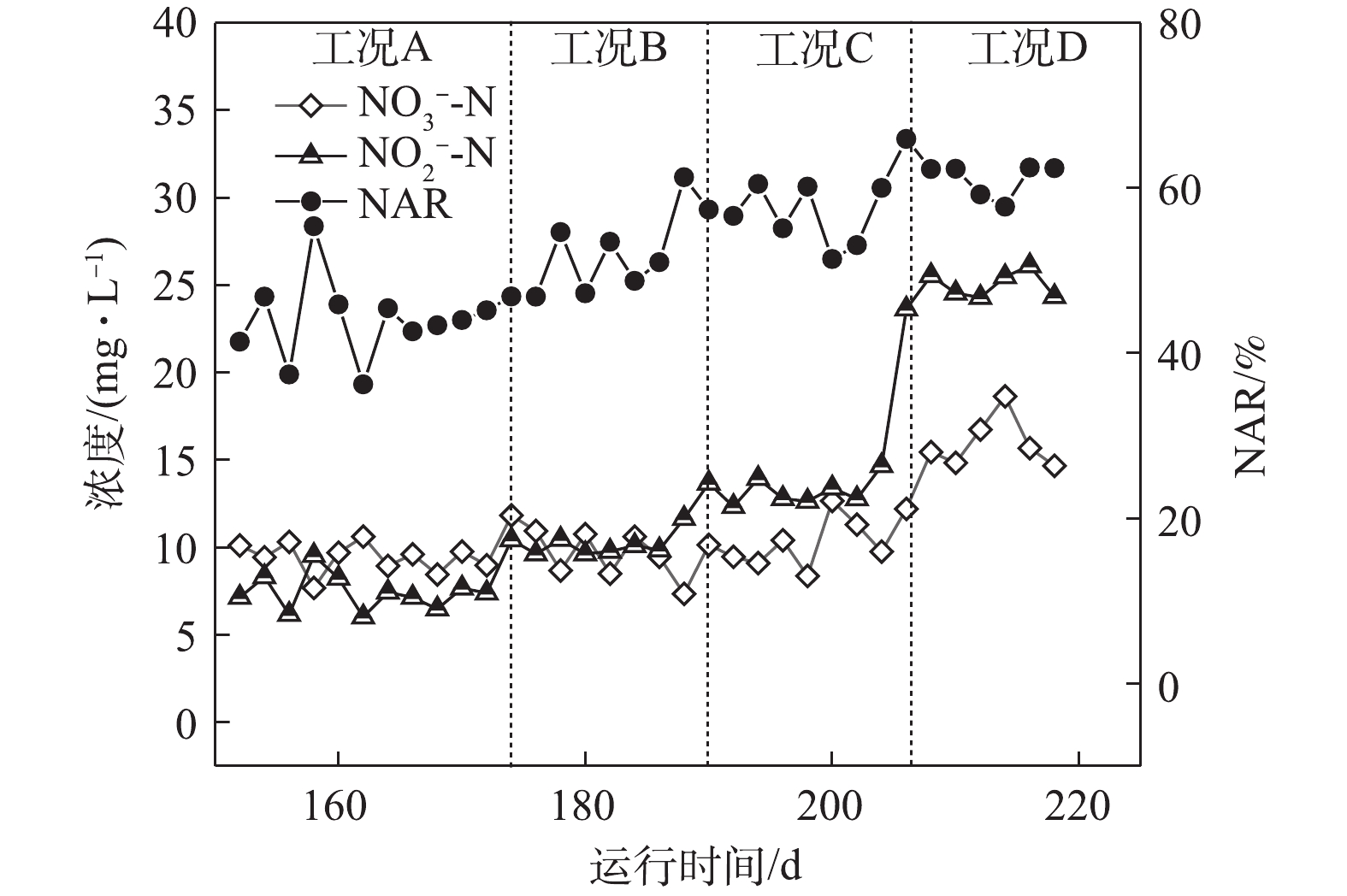
如图2所示,在工况A、B、C和D下,MBR反应器内对应的平均亚硝酸盐积累率分别为43.9%、51.3%、56.8%和61.7%。在工况A下,
NH+4 -N转化率高达98%;随着反应的持续进行,较低的NH+4 -N负荷使得AOBs的基质逐步缺失,同时较低的NH+4 -N浓度也使得FA值较低,因此,MBR反应器对NOBs的抑制效果较差,具有较强活性的NOBs会将NO−2 -N继续氧化为NO−3 -N,从而使得NAR较低,所以,保证充足的氨氮浓度和抑制NOBs的生长是短程硝化稳定运行的关键所在[6]。随着基质浓度的上升,NH+4 -N负荷逐渐升高,在工况C和工况D下,出水NH+4 -N出现少量剩余,这说明AOBs基质浓度充足,且其活性逐渐增强。本研究中,短程硝化稳定运行的因素主要归于以下几点:首先是合适的SRT。MULDER等[7]的研究表明,在14 ℃以上时,AOBs和NOBs的世代周期分别为8~36 h和12~59 h。因此,将污泥龄控制在AOBs与NOBs最小世代周期内,NOBs就会被逐渐淘洗掉,使AOBs成为优势菌群,本研究通过排除泥水混合液将泥龄控制在15 d。其次是适宜的温度。在20 ℃时,AOBs和NOBs的比增长速率μmax分别为0.801 d−1和0.788 d−1;当温度低于20 ℃时,AOBs的μmax小于NOBs,大于20 ℃时则相反[8]。再次是维持较低的DO浓度。有研究[9]表明,AOBs的氧饱和系数为0.2~0.4 mg·L−1,NOBs的氧饱和系数为1.2~1.6 mg·L−1,因此,控制DO浓度是实现短程硝化的限制性因素,本研究将DO浓度基本维持在0.7 mg·L−1左右,由于NOBs的活性长期受到抑制,故使得NAR逐渐升高。最后是控制较高的FA值,从而抑制NOBs的生长[10]。有研究[11]表明,当FA浓度为0.1~1.0 mg·L−1时,NOBs则会受到其抑制作用,FA浓度达到6 mg·L−1时,则NOBs的生长代谢则几乎被完全抑制,而AOBs对FA的受抑制范围则为10~150 mg·L−1。在工况A、B、C和D下,FA的浓度分别为4.48、6.72、8.96和11.19 mg·L−1,在工况D下,
NH+4 -N浓度为150 mg·L−1,NAR稳定在60%以上,通过FA选择性抑制NOBs,可使系统短程硝化高效稳定运行。2.2 系统对COD的去除特性
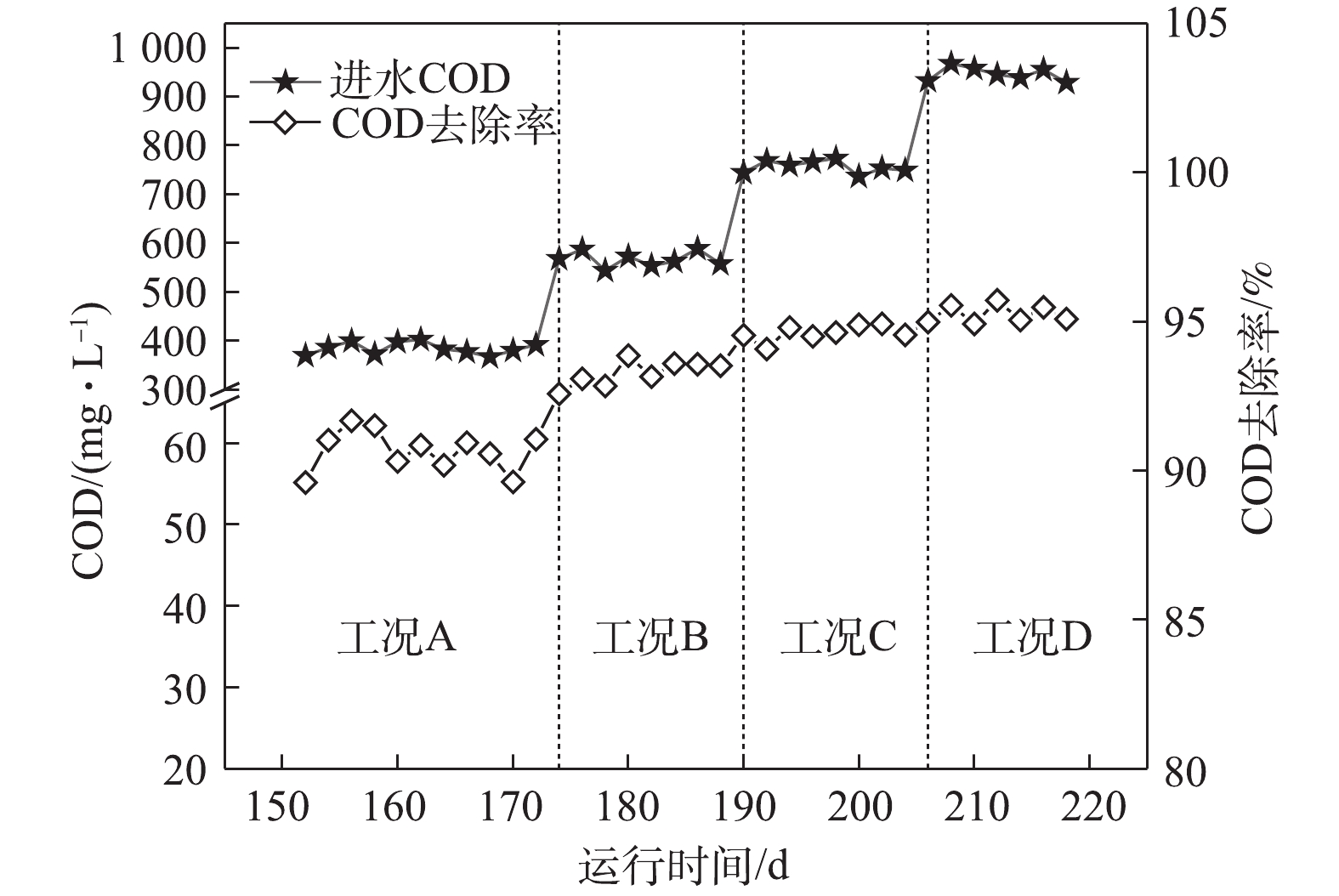
图3为系统COD去除情况。由图3可知,在工况A、B、C和D下,系统对COD的去除率分别为90.6 %、93.3 %、94.6 %和95.3%。随着进水COD逐渐上升,其对应的ABR容积负荷(以COD计)分别为1.02、1.53、2.04和2.55 kg·(m3·d)−1。在工况A和工况B时,对COD去除占主导作用的是前3个隔室,主要是通过A1厌氧隔室的水解酸化作用产生VFA,使DPBs利用优质碳源进行厌氧释磷完成对COD的去除。而随着基质浓度的升高,容积负荷(VLR)的升高使得前3个隔室的去除效率下降。在工况C和工况D时,对COD去除作用的功能隔室则逐渐后移,A4厌氧隔室通过产甲烷菌对剩余的COD进一步去除,剩余耗氧有机污染物(以COD计)则通过常规异养反硝化菌在缺氧段利用硝化液中的
NO−x -N被去除,至此大部分耗氧有机污染物(以COD计)已在ABR反应器去除,ABR反应器的出水分别为35.9、38.1、40.8和45.1 mg·L−1。从工况A至工况D,虽然COD呈梯度上升,但前端厌氧及缺氧隔室对NH+4 -N仅有少量同化作用而去除,而经过前端ABR反应器对COD较高的去除率,使得进入MBR反应器的C/N值逐渐降低。张婷等[12]研究表明,当C/N=1时,有利于短程硝化的运行,而过高的C/N值会导致普通异养菌的快速增殖,进而降低硝化反应的速率。本研究中进入MBR反应器的C/N值低于1,因此,C/N不会成为影响短程硝化的限制性因素。2.3 系统对氮的去除特性
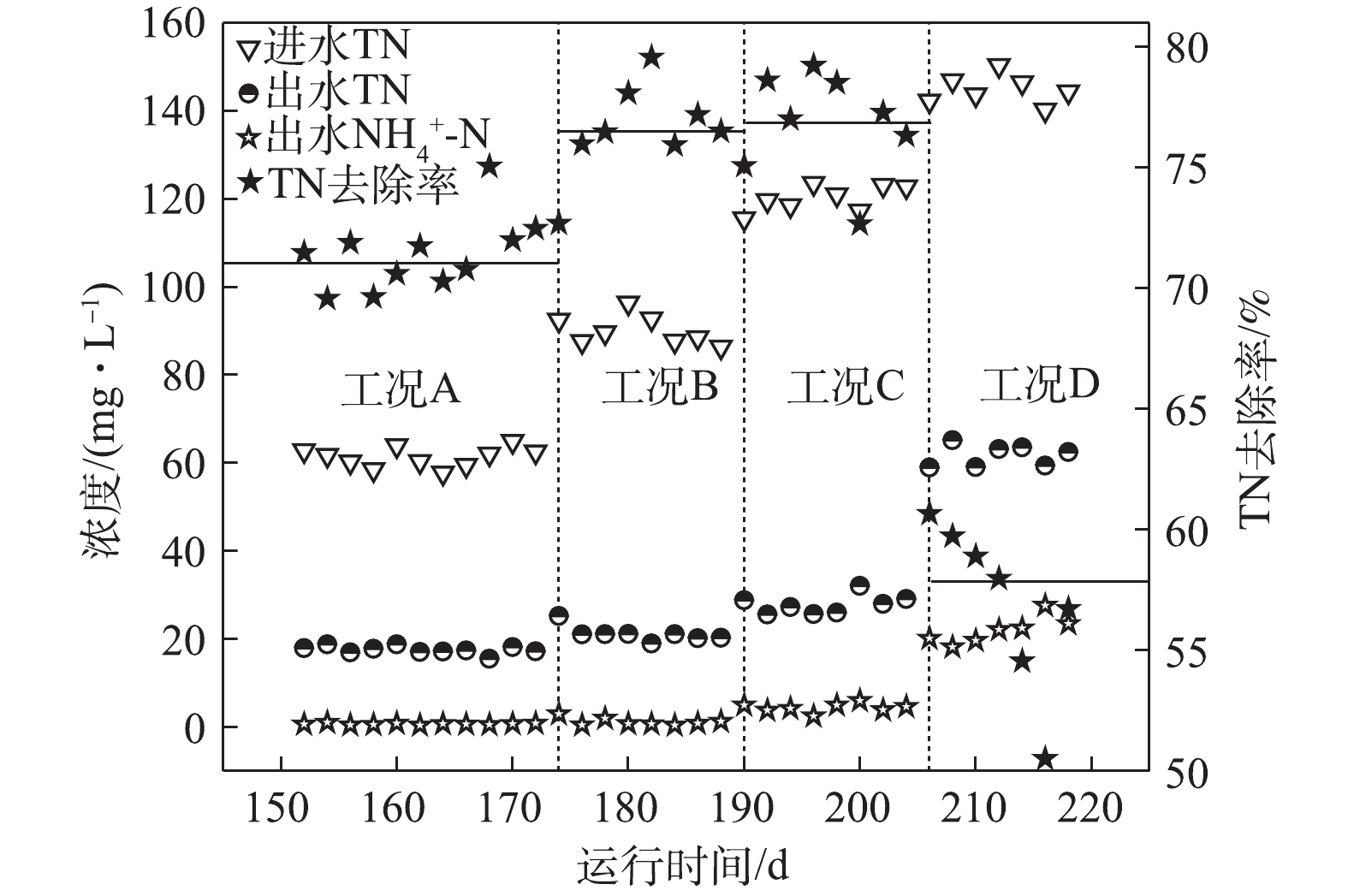
如图4所示,在工况A、B、C和D下,TN平均去除率分别为71.4%、76.6%、76.8%和56.3%。在工况A下,基质浓度较低,大部分耗氧有机污染物(以COD计)在缺氧段之前已经被完全去除,因此,系统脱氮则完全由DPBs主导,此时脱氮效率相对较低。在工况B和工况C条件下,虽然NAR稳定在55%以上,但由于回流硝化液中
NO−2 -N浓度较低,尚未达到抑制缺氧吸磷的阈值,所以系统反硝化除磷脱氮效果依然很好,并且有剩余耗氧有机污染物(以COD计)参与了常规反硝化的作用,使得脱氮效率逐步提高。同时,GAOs可能对脱氮也具有一定的贡献。GAOs与PAOs具有相似的代谢机制,在反硝化除磷过程中,GAOs的增殖始终伴随着PAOs的富集[13]。有研究者[14]报道,在缺氧条件下,GAOs可以进行与有氧条件下相同的代谢,GAOs在厌氧环境下形成的PHAs,在缺氧中利用其完成糖原的再生以及脱氮的作用,在脱氮过程中发挥了重要作用。在工况D条件下,由于基质浓度的升高使得MBR反应器内的NAR较高,进而使得回流硝化液中NO−2 -N浓度超过了DPBs的耐受限度,导致反硝化吸磷效果较低,从而降低了脱氮的效能。而较高的基质浓度使得AOBs对氨氮的转化率也下降至84.2%,测定到的出水NH+4 -N平均浓度为22.92 mg·L−1,因此,一定的基质浓度范围有利于系统的脱氮效能。2.4 系统对磷的去除特性
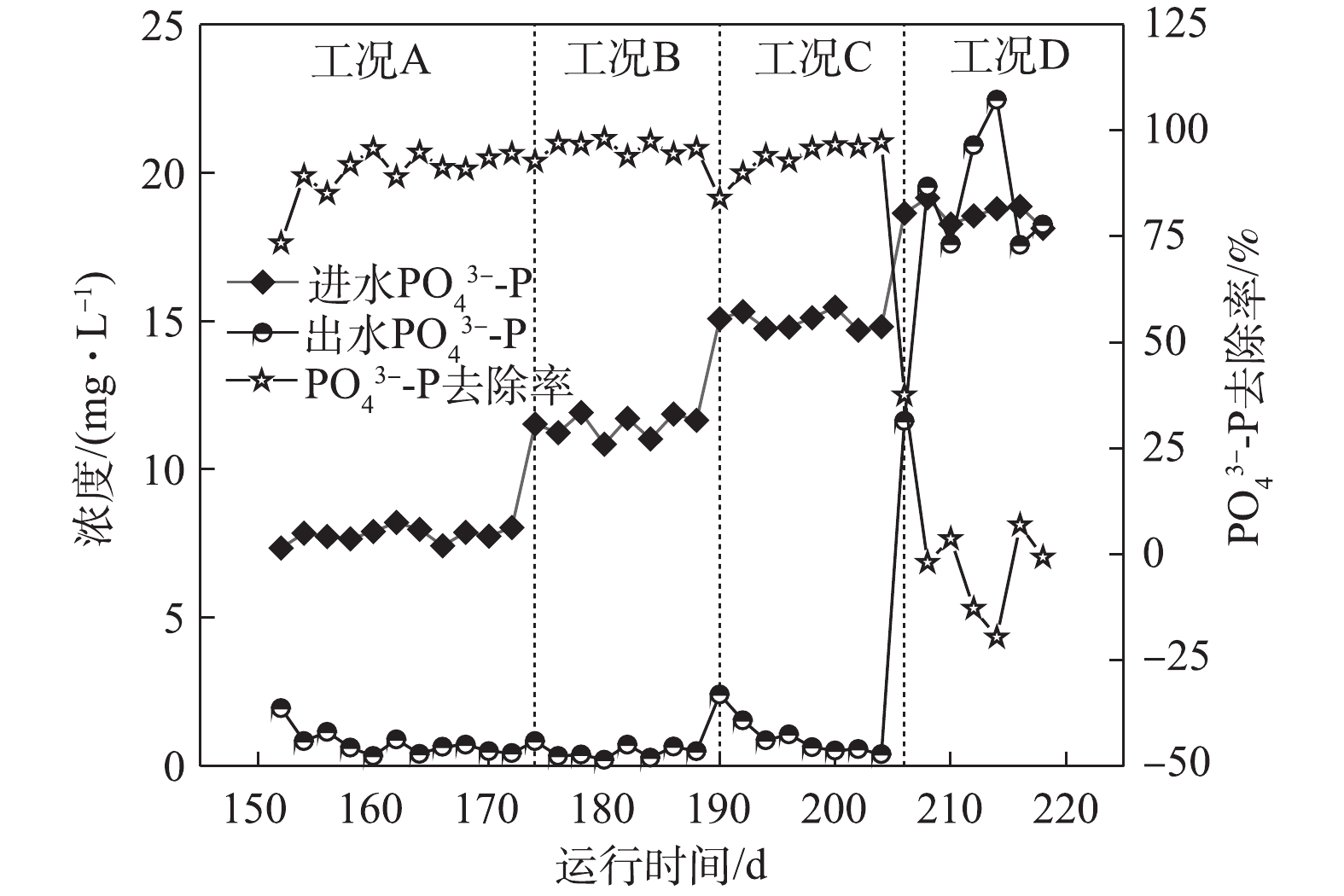
如图5所示,在工况A、B、C和D下,对应的ABR反应器中容积负荷(以
PO3−4 -P计)分别为0.021、0.032、0.043和0.053 kg·(m3·d)−1,系统对磷的平均去除率分别为89.96%、95.74%、93.3%和1.84%。在工况A条件下,MBR反应器NAR平均为43.9%,且系统除磷率达到89.96%。这表明NO−2 -N能够作为电子受体实现除磷,与ZHANG等[15]的研究结论一致,即低浓度的NO−2 -N并未抑制反硝化吸磷作用的进行。RUBIO-RINCÓN等[13]使用16SrRNA基因扩增子测序和多聚磷酸盐激酶基因(ppk1)作为遗传标记,结果表明,“Candidatus Accumulibacter ”分为2个主要的支系(PAO Ⅰ和PAO Ⅱ)。同时,通过宏基因组分析表明,PAO II的宏基因组缺乏呼吸硝酸盐还原酶(nar),但具有利用亚硝酸盐还原为氮气的能力,这表明PAO Ⅱ不能利用硝酸盐进行除磷,而PAO Ⅰ则能够利用O2,NO−2 -N及NO−3 -N。在工况B条件下,虽然ABR进水磷负荷升高至0.032 kg·(m3·d)−1,MBR反应器平均NAR为51.3%,
NO−2 -N平均浓度达到10.60 mg·L−1,但出水PO3−4 -P依旧稳定在0.5 mg·L−1左右,PO3−4 -P的去除率高达95.74%,表明DPBs对NO−2 -N的耐受并未达到极限。在工况C条件下,随着容积负荷的升高,出水NO−2 -N平均浓度为13.28 mg·L−1,回流硝化液中NO−2 -N浓度随之升高,这对DPBs有一定的影响,在第190天时,出水PO3−4 -P增至2.41 mg·L−1。随着ABR反应器内反硝化除磷功能区对DPBs的驯化,出水平均PO3−4 -P稳定至1.0 mg·L−1以下。在工况D下,回流NO−x -N中NO−2 -N/NO−3 -N为0.617,出水NO−2 -N平均浓度高达24.9 mg·L−1,磷去除率瞬间下降,甚至出现了负值,磷吸收阶段受到严重的抑制作用。诸多研究[16-18]表明,亚硝酸质子化产物—游离亚硝酸(FNA)是缺氧吸磷真正的抑制剂,而并非是亚硝酸盐的影响所致。在工况D条件下,FNA为0.001 3 mg·L−1,低于ZHOU等[19]所报道的FNA对缺氧磷吸收完全抑制的浓度。FNA对吸磷的抑制作用主要是通过对微生物新陈代谢的影响得以实现:首先,FNA影响ATP的合成,能够提高质子透过膜的透过性,导致质子推动力的效果变差,从而影响了胞内聚磷的合成[17];其次,FNA抑制反硝化酶的活性以及缺氧过程PHA的氧化,ZENG等[18]的研究表明,在缺氧条件下,FNA完全抑制吸磷时,PHA首先被用于NO−2 -N的还原从而实现解毒作用,而不是作为吸磷的能量来源被DPBs分解。3. 结论
1)控制ABR反应器HRT为9 h、C/N/P值不变、提高进水基质浓度,在此条件下,MBR反应器在工况A、B、C和D下平均亚硝酸盐积累率分别为43.9%、51.3%、56.8%和61.7%;通过SRT、DO浓度、温度及FA的协同作用稳定短程硝化的运行,控制FA选择性抑制NOBs的功能,可使AOBs逐渐成为优势菌群。
2)随着基质浓度的上升,负荷的升高使得前3隔室的COD去除率下降。在工况C和工况D下,对COD去除作用的功能隔室则逐渐后移,剩余耗氧有机污染物(以COD计)则通过常规异养反硝化菌在缺氧段利用硝化液中的
NO−x -N被去除;同时基质浓度的上升也提高了脱氮率,在一定的基质浓度范围内有利于系统的处理效能。3) FNA是缺氧吸磷真正的抑制剂。在FNA为0.001 3 mg·L−1时,磷去除率骤降,缺氧吸磷受到严重的抑制作用,这主要是因为FNA影响了ATP的合成,并且FNA抑制反硝化酶的活性以及缺氧过程中PHA的氧化。
-
[1] [2] 梁忠. 中国抗生素52%为兽用[J]. 中国禽业导刊, 2015(12):75. LIANG Z. In China 52% antibioitcs used for animals[J]. Guide to Chinese Poultry,2015 (12):75(in Chinese).
[3] 廖新俤, 蒋骥, 吴银宝, 等. 猪场使用药物饲料添加剂对环境的影响[J]. 家畜生态, 2001, 22(1):13-15. LIAO X D, JIANG J, WU Y B, et al. The effect of using drug feed additive on environment in pig farm[J]. Ecology of Domestic Animal, 2001, 22(1):13-15(in Chinese).
[4] JOY S R, BARTELT-HUNT S L, SNOW D D, et al. Fate and transport of antimicrobials and antimicrobial resistance genes in soil and runoff following land application of swine manure slurry[J]. Environmental Science & Technology, 2013, 47(21):12081-12088. [5] ZHANG H, LUO Y, WU L, et al. Residues and potential ecological risks of veterinary antibiotics in manures and composts associated with protected vegetable farming[J]. Environmental Science and Pollution Research, 2015, 22(8):5908-5918. [6] HOU J, WAN W, MAO D, et al. Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soils of Northern China[J]. Environmental Science and Pollution Research, 2015, 22(6):4545-4554. [7] LI W, SHI Y, GAO L, et al. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China[J]. Chemosphere, 2012, 89(11):1307-1315. [8] HUANG X, LIU C, LI K, et al. Occurrence and distribution of veterinary antibiotics and tetracycline resistance genes in farmland soils around swine feedlots in Fujian Province, China[J]. Environ Sci Pollut Res Int, 2013, 20(12):9066-9074. [9] LIU B, LI Y, ZHANG X, et al. Effects of composting process on the dissipation of extractable sulfonamides in swine manure[J]. Bioresource Technology, 2015, 175:284-290. [10] HUANG X, LIU C, LI K, et al. Performance of vertical up-flow constructed wetlands on swine wastewater containing tetracyclines and tet genes[J]. Water Research, 2015, 70:109-117. [11] SARMAH A K, MEYER M T, BOXALL A B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment[J]. Chemosphere, 2006, 65(5):725-759. [12] PAN X, QIANG Z, BEN W, et al. Residual veterinary antibiotics in swine manure from concentrated animal feeding operations in Shandong Province, China[J]. Chemosphere, 2011, 84(5):695-700. [13] SELVAM A, ZHAO Z Y, WONG J W C. Composting of swine manure spiked with sulfadiazine, chlortetracycline and ciprofloxacin[J]. Bioresource Technology, 2012, 126:412-417. [14] BAO Y Y, ZHOU Q X, GUAN L Z, et al. Depletion of chlortetracycline during composting of aged and spiked manures[J]. Waste Management, 2009, 29(4):1416-1423. [15] 王桂珍, 李兆君, 张树清, 等. 土霉素在鸡粪好氧堆肥过程中的降解及其对相关参数的影响[J]. 环境科学, 2013, 34(2):795-803. WANG G Z, LI Z J, ZHANG S Q, et al. Degradation of oxytetracycline in chicken feces aerobic-composting and its effects on their related parameters[J]. Environmental Science, 2013, 34(2):795-803(in Chinese).
[16] ARIKAN O A, MULBRY W, RICE C. Management of antibiotic residues from agricultural sources:use of composting to reduce chlortetracycline residues in beef manure from treated animals[J]. Journal of Hazardous Materials, 2009, 164(2):483-489. [17] DOLLIVER H, GUPTA S, NOLL S. Antibiotic degradation during manure composting[J]. J Environ Qual, 2008, 37(3):1245-1253. [18] MUNARETTO J S, YONKOS L, AGA D S. Transformation of ionophore antimicrobials in poultry litter during pilot-scale composting[J]. Environmental Pollution, 2016, 212:392-400. [19] RAMASWAMY J, PRASHER S O, PATEL R M, et al. The effect of composting on the degradation of a veterinary pharmaceutical[J]. Bioresource Technology, 2010, 101(7):2294-2299. [20] QIU J, HE J, LIU Q, et al. effects of conditioners on sulfonamides degradation during the aerobic composting of animal manures[J]. Procedia Environmental Sciences, 2012, 16:17-24. [21] SELVAM A, ZHAO Z Y, LI Y C, et al. Degradation of tetracycline and sulfadiazine during continuous thermophilic composting of pig manure and sawdust[J]. Environmental Technology, 2013, 34(16):2433-2441. [22] HU Z H, LIU Y L, CHEN G W, et al. Characterization of organic matter degradation during composting of manure-straw mixtures spiked with tetracyclines[J]. Bioresource Technology, 2011, 102(15):7329-7334. [23] 周孟津, 张榕林, 蔺金印. 沼气实用技术[M].北京:化学工业出版社, 2004. ZHOU M J, ZHANG R L, LIN J Y. Practical technology of marsh gas[M]. Beijing:Chemical Industry Press,2004(in Chinese). [24] 彭武厚, 胡文英, 李新吾. 厌氧消化法处理畜禽粪的研究[J]. 中国沼气, 1998, 16(1):15-17. PENG W H, HU W Y, LI X W. Studies on anaerobic digestion of livestock excrement[J]. China Biogas,1998, 16(1):15-17(in Chinese).
[25] ÁLVAREZ J, OTERO L, LEMA J, et al. The effect and fate of antibiotics during the anaerobic digestion of pig manure[J]. Bioresource Technology, 2010, 101(22):8581-8586. [26] MOHRING S A, STRZYSCH I, FERNANDES M R, et al. Degradation and elimination of various sulfonamides during anaerobic fermentation:A promising step on the way to sustainable pharmacy?[J]. Environmental Science & Technology, 2009, 43(7):2569-2574. [27] SHI J, LIAO X, WU Y, et al. Effect of antibiotics on methane arising from anaerobic digestion of pig manure[J]. Animal feed Science and Technology, 2011, 166:457-463. [28] JOY S R, LI X, SNOW D D, et al. Fate of antimicrobials and antimicrobial resistance genes in simulated swine manure storage[J]. Science of the Total Environment, 2014, 481:69-74. [29] VAREL V, WELLS J, SHELVER W, et al. Effect of anaerobic digestion temperature on odour, coliforms and chlortetracycline in swine manure or monensin in cattle manure[J]. Journal of Applied Microbiology, 2012, 112(4):705-715. [30] WITHEY J M, MUGO S M, ZHOU T, et al. Depletion of hormones and antimicrobials in cattle manure using thermophilic anaerobic digestion[J]. Journal of Chemical Technology and Biotechnology, 2015, 91(9):2404-2411. [31] TURKER G, AYDIN S, AKYOL Ç, et al. Changes in microbial community structures due to varying operational conditions in the anaerobic digestion of oxytetracycline-medicated cow manure[J]. Applied Microbiology and Biotechnology, 2016:1-11. [32] AL-AHMAD A, DASCHNER F, K MMERER K. Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole and inhibition of waste water bacteria[J]. Archives of Environmental Contamination and Toxicology, 1999, 37(2):158-163. [33] CHEN Y, ZHANG H, LUO Y, et al. Occurrence and dissipation of veterinary antibiotics in two typical swine wastewater treatment systems in east China[J]. Environmental Monitoring and Assessment, 2012, 184(4):2205-2217. [34] WEI R, GE F, HUANG S, et al. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China[J]. Chemosphere, 2011, 82(10):1408-1414. [35] HO Y B, ZAKARIA M P, LATIF P A, et al. Occurrence of veterinary antibiotics and progesterone in broiler manure and agricultural soil in Malaysia[J]. Science of the Total Environment, 2014, 488:261-267. [36] BARTELT-HUNT S, SNOW D D, DAMON-POWELL T, et al. Occurrence of steroid hormones and antibiotics in shallow groundwater impacted by livestock waste control facilities[J]. Journal of Contaminant Hydrology, 2011, 123(3):94-103. [37] TONG L, LI P, WANG Y, et al. Analysis of veterinary antibiotic residues in swine wastewater and environmental water samples using optimized SPE-LC/MS/MS[J]. Chemosphere, 2009, 74(8):1090-1097. [38] SANCHEZ E, BORJA R, TRAVIESO L, et al. Effect of influent substrate concentration and hydraulic retention time on the performance of down-flow anaerobic fixed bed reactors treating piggery wastewater in a tropical climate[J]. Process Biochemistry, 2005, 40(2):817-829. [39] DENG L W, ZHENG P, CHEN Z A. Anaerobic digestion and post-treatment of swine wastewater using IC-SBR process with bypass of raw wastewater[J]. Process Biochemistry, 2006, 41(4):965-969. [40] MONTUELLE B, GOILLARD J, HY J B L. A combined anaerobic-aerobic process for the co-treatment of effluents from a piggery and a cheese factory[J]. Journal of Agricultural Engineering Research, 1992, 51:91-100. [41] CASTRILLON L, FERN NDEZ-NAVA Y, MARANON E, et al. Anoxic-aerobic treatment of the liquid fraction of cattle manure[J]. Waste Management, 2009, 29(2):761-766. [42] BEN W, QIANG Z, PAN X, et al. Removal of veterinary antibiotics from sequencing batch reactor (SBR) pretreated swine wastewater by Fenton's reagent[J]. Water Research, 2009, 43(17):4392-4402. [43] OTHMAN I, ANUAR A N, UJANG Z, et al. Livestock wastewater treatment using aerobic granular sludge[J]. Bioresource Technology, 2013, 133:630-634. [44] WHANG G, CHO Y, PARK H, et al. The removal of residual organic matter from biologically treated swine wastewater using membrane bioreactor process with powdered activated carbon[J]. Water Science and Technology, 2004, 49(5-6):451-457. [45] PRADO N, OCHOA J, AMRANE A. Zero Nuisance Piggeries:Long-term performance of MBR (membrane bioreactor) for dilute swine wastewater treatment using submerged membrane bioreactor in semi-industrial scale[J]. Water Research, 2009, 43(6):1549-1558. [46] 庄榆佳, 高阳俊, 邓玉君, 等. 微生物固化曝气技术对养殖废水的深度处理[J]. 环境化学, 2015, 34(7):1356-1362. ZHUANG Y J, GAO J Y, DENG Y J, et al.Advanced treatment of swine wastewater by the immobilizedmicroorganism and aeration technology[J]. Environmental Chemistry, 2015, 34(7):1356-1362(in Chinese).
[47] ELMOLLA E, CHAUDHURI M. Optimization of Fenton process for treatment of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution[J]. Journal of Hazardous Materials, 2009, 170(2):666-672. [48] P REZ-MOYA M, GRAELLS M, CASTELLS G, et al. Characterization of the degradation performance of the sulfamethazine antibiotic by photo-Fenton process[J]. Water Research, 2010, 44(8):2533-2540. [49] BEN W, QIANG Z, PAN X, et al. Degradation of veterinary antibiotics by ozone in swine wastewater pretreated with sequencing batch reactor[J]. Journal of Environmental Engineering, 2011, 138(3):272-277. [50] 李文君, 蓝梅, 彭先佳. UV/H2O2 联合氧化法去除畜禽养殖废水中抗生素[J]. 环境污染与防治, 2011, 33(4):25-28. LI W J, LAN M, PENG X J. Removal of antibiotics from swine wastewater by UV/H2O2 combined oxidation[J]. Environmental Pollution & Control,2011, 33(4):25-28(in Chinese).
[51] [52] 张德莉, 黄应平, 罗光富, 等. Fenton及Photo-Fenton反应研究进展[J]. 环境化学, 2006, 25(2):121-127. ZHANG D L, HUANG Y P, LUO G F,et al. Research process of Fenton and Photo-Fenton reaction[J]. Envionmental Chemistry, 2006, 25(2):121-127(in Chinese).
[53] 郭思, 刘燕, 杨楠桢, 等. Fenton氧化法处理生物性污染废水[J]. 环境化学, 2009, 28(4):487-491. GUO S, LIU Y, YANG N Z et al. Treatment of bio-polluted wastewater by Fenton oxidation[J]. Envionmetal Chemistry, 2009, 28(4):487-491(in Chinese).
[54] KNIGHT R L, PAYNE V W, BORER R E, et al. Constructed wetlands for livestock wastewater management[J]. Ecological Engineering, 2000, 15(1):41-55. [55] LIU L, LIU C X, ZHENG J Y, et al. Elimination of veterinary antibiotics and antibiotic resistance genes from swine wastewater in the vertical flow constructed wetlands[J]. Chemosphere, 2013, 91(8):1088-1093. [56] LIU L, LIU Y H, WANG Z, et al. Behavior of tetracycline and sulfamethazine with corresponding resistance genes from swine wastewater in pilot-scale constructed wetlands[J]. Journal of Hazardous Materials, 2014, 278:304-310. [57] FERNANDES J P, ALMEIDA C M R, PEREIRA A C, et al. Microbial community dynamics associated with veterinary antibiotics removal in constructed wetlands microcosms[J]. Bioresource Technology, 2015, 182:26-33. [58] CARVALHO P N, ARAUJO J L, MUCHA A P, et al. Potential of constructed wetlands microcosms for the removal of veterinary pharmaceuticals from livestock wastewater[J]. Bioresource Technology, 2013, 134:412-416. [59] XIAN Q, HU L, CHEN H, et al. Removal of nutrients and veterinary antibiotics from swine wastewater by a constructed macrophyte floating bed system[J]. Journal of Environmental Management, 2010, 91(12):2657-2661. [60] AVILA C, MATAMOROS V, REYES-CONTRERAS C, et al. Attenuation of emerging organic contaminants in a hybrid constructed wetland system under different hydraulic loading rates and their associated toxicological effects in wastewater[J]. Science of the Total Environment, 2014, 470:1272-1280. [61] DAN A, YANG Y, DAI Y N, et al. Removal and factors influencing removal of sulfonamides and trimethoprim from domestic sewage in constructed wetlands[J]. Bioresource Technology, 2013, 146:363-370. [62] YAN Q, FENG G Z, GAO X, et al. Removal of pharmaceutically active compounds (PhACs) and toxicological response of Cyperus alternifolius exposed to PhACs in microcosm constructed wetlands[J]. Journal of Hazardous Materials, 2016, 301:566-575. [63] THIELE-BRUHN S. Pharmaceutical antibiotic compounds in soils-A review[J]. Journal of Plant Nutrition and Soil Science, 2003, 166(2):145-167. [64] CHEE-SANFORD J C, MACKIE R I, KOIKE S, et al. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste[J]. J Environ Qual, 2009, 38(3):1086-1108. [65] WU X, WEI Y, ZHENG J, et al. The behavior of tetracyclines and their degradation products during swine manure composting[J]. Bioresource Technology, 2011, 102(10):5924-5931. 期刊类型引用(31)
1. 童林. 农林废弃物处理抗生素废水的研究进展. 中国资源综合利用. 2024(01): 113-116 .  百度学术
百度学术
2. 陈言鑫,王健,孟海波,丁京涛,周海宾,程红胜,徐鹏翔,张曦,程琼仪,马双双,张朋月,陈云峰. 不同反应器好氧堆肥工艺对猪粪中磺胺甲噁唑降解效果研究. 黑龙江畜牧兽医. 2024(05): 1-7 .  百度学术
百度学术
3. 常潇,韩宇杰,尚丽元,钟荣珍. 种养循环系统中典型有害物质对动物的危害研究进展. 动物营养学报. 2024(07): 4081-4093 .  百度学术
百度学术
4. 阚泽鑫,朱宁,龙玉娇,靳红梅. 高温预处理联合生物炭对猪粪堆肥中抗生素消减和重金属钝化的促进作用. 农业环境科学学报. 2023(04): 879-890 .  百度学术
百度学术
5. 谢晓杰,许双燕,王文凡,杨健,赵卓群,王敏,郑华宝. 畜禽养殖废弃物中抗生素的微生物降解研究进展. 浙江农业学报. 2023(08): 1975-1992 .  百度学术
百度学术
6. 韩岩松,郑锌,黄均明,保万魁,刘红芳,刘蜜,王旭. 超高效液相色谱-串联质谱法同时测定畜禽粪便中34种抗生素含量. 中国土壤与肥料. 2022(02): 216-229 .  百度学术
百度学术
7. 万辰,陈思玮,马瑛骏,张克强,王风,沈仕洲. 抗生素残留对施用牛粪土壤氮素矿化的影响及amoA、nxrA基因的响应. 环境污染与防治. 2022(12): 1616-1621+1627 .  百度学术
百度学术
8. 谢丽,张艺蝶,朱雯喆,何莹莹. 林可霉素和3种大环内酯类抗生素对厌氧消化的影响. 同济大学学报(自然科学版). 2021(02): 254-263 .  百度学术
百度学术
9. 王吉平,苏天明,张野,王瑾,何铁光. 基于文献计量学的农业废弃物资源化利用研究现状及态势分析. 湖南生态科学学报. 2021(01): 58-69 .  百度学术
百度学术
10. 陈冠益,刘环博,李健,颜蓓蓓,董磊. 抗生素菌渣处理技术研究进展. 环境化学. 2021(02): 459-473 .  本站查看
本站查看
11. 王晓洁,赵蔚,张志超,程和发,陶澍. 兽用抗生素在土壤中的环境行为、生态毒性及危害调控. 中国科学: 技术科学. 2021(06): 615-636 .  百度学术
百度学术
12. 燕翔,吴生平,王都留,张少飞,尚芳圆. 畜禽养殖业废水危害及处理技术研究进展. 黑龙江畜牧兽医. 2021(15): 36-39 .  百度学术
百度学术
13. 杜实之. 环境中抗生素的残留、健康风险与治理技术综述. 环境科学与技术. 2021(09): 37-48 .  百度学术
百度学术
14. 张文婕,杨莉莉,王金花,朱鲁生,王军,毛书帅. 三种抗生素与铜复合污染对土壤过氧化氢酶活性的影响. 农业资源与环境学报. 2020(01): 135-143 .  百度学术
百度学术
15. 徐磊,叶雪平,郝贵杰,盛鹏程,周冬仁,孙博怿,张海琪. 苕溪表层水体典型抗生素污染特征与生态风险评价. 现代农业科技. 2020(07): 180-183+187 .  百度学术
百度学术
16. 车艳丽,程瑞锋,张雅婷,杨其长. 酒糟沼渣基质对番茄生长及其品质的影响. 山东农业科学. 2020(04): 98-105 .  百度学术
百度学术
17. 晏广,邱兆富,曹国民,孙贤波,黄晓霞,卞晓彤. A/O系统处理低C/N奶牛场废水中的抗生素. 环境工程学报. 2020(07): 1817-1826 .  百度学术
百度学术
18. 李龙威,张頔,徐建玲. 基于文献计量的农业废弃物研究现状与发展趋势分析. 东北师大学报(自然科学版). 2020(03): 150-156 .  百度学术
百度学术
19. 余佩瑶,刘寒冰,邓艳玲,薛南冬. 畜禽粪便中抗生素污染特征及堆肥化去除研究进展. 环境化学. 2019(02): 334-343 .  本站查看
本站查看
20. 张昱,田甜甜,马春萌,杨敏. 生物处理过程次生风险的产生与控制研究进展. 生物产业技术. 2019(02): 28-37 .  百度学术
百度学术
21. 吴爽爽,解诗雨,李佳佳,刘香檬,莫秋霞,田佳,芦帅,吴楠. 畜禽粪便中常见抗生素去除的研究进展. 天津农学院学报. 2019(02): 89-92 .  百度学术
百度学术
22. 强虹,杨祎楠,李娜,宋亚楠,李玉友. 金霉素浓度对鸡粪中温厌氧消化特性及抗生素降解的影响. 农业工程学报. 2019(10): 181-190 .  百度学术
百度学术
23. 陈金峰,刘海林,邹春萍,刘可星,张佩霞,孙映波. 2种植物浮床对含抗生素养殖废水的净化效果. 水土保持通报. 2019(03): 137-143 .  百度学术
百度学术
24. 涂敏. 规模化养猪场粪污处理与综合利用综述. 安徽农学通报. 2019(15): 139-143 .  百度学术
百度学术
25. 亓爱杰,宇凌. 益生菌在我国养殖业的应用及改进建议. 饲料博览. 2019(08): 14-18 .  百度学术
百度学术
26. 鲍雨,姜钰,张军,王敦球. 桂林市城市污泥和污泥堆肥中氟喹诺酮类抗生素调查研究. 给水排水. 2019(S1): 194-196+219 .  百度学术
百度学术
27. 王莹,王婧臻,楚杰. 活性复合菌饲料添加剂对肉鸡肠道酶活及生长性能的影响. 饲料研究. 2019(12): 44-47 .  百度学术
百度学术
28. 杨月琴,钟成华. 垂直流人工湿地去除布洛芬和罗红霉素的影响因素分析. 环境化学. 2019(12): 2780-2788 .  本站查看
本站查看
29. 张鹏飞,刘晓文,李杰,吴颖欣,刘沙沙,王勇. 养殖废水中抗生素去除处理工艺的研究现状. 净水技术. 2018(04): 60-65+95 .  百度学术
百度学术
30. 邱美珍,谢菊兰,任慧波,杜丽飞,冯小花,周望平,蔡文杰,朱静波,王红兵. 畜禽粪污中残留抗生素降解方法进展. 激光生物学报. 2018(04): 308-312 .  百度学术
百度学术
31. 张宾朋,韩秀丽,方书起,常春. 响应面法优化脱硅稻壳基活性炭对恩诺沙星的吸附. 郑州大学学报(工学版). 2018(06): 64-68 .  百度学术
百度学术
其他类型引用(45)
-

 点击查看大图
点击查看大图
计量
- 文章访问数: 3095
- HTML全文浏览数: 2993
- PDF下载数: 816
- 施引文献: 76





 下载:
下载: