-
生物质能作为世界能源消费体系中的第四大能源,仅次于煤炭、石油和天然气,近年来得到了国家的重点鼓励和扶持,在未来能源结构中,生物质能作为可再生能源将发挥不可替代的作用[1-2]。近十年来,我国畜禽粪便年产量基本稳定在37亿吨,畜禽粪便中含有丰富的水分(50.5%)、有机质(25.5%)、氮(1.63%)、磷(1.54%)、硫化氢以及大量的病原菌等,由于产量巨大且综合利用率不足,是造成环境污染的主要污染源之一[3],主要表现为易产生面源污染、粪便中病原体释放、气味恶劣等[4-5],存在分散性、累积性和模糊性等特点,控制难度较大[6]。然而其作为一种生物质能,得到有效利用可以缓解能源紧张带来的压力并减少其对环境造成的污染。厌氧消化作为畜禽粪便等有机污染物的处置方法之一,可以将有机物转化为CH4、CO2和各类小分子脂肪酸,实现资源的进一步利用[7]。
温度作为厌氧消化的主要影响因素之一,将厌氧消化划分为常温厌氧消化(15~25 ℃)、中温厌氧消化(30~40 ℃)和高温厌氧消化(50~60 ℃)[8],由于常温厌氧消化甲烷产量和有机物降解效率较低,中高温厌氧消化目前应用较为广泛。近年来很多研究表明,在高温厌氧消化过程中,有机物的降解效率、甲烷产量以及挥发性脂肪酸(VFAs)的去除率均高于中温厌氧消化[9-10]。KJERSTADIUS et al[11]通过在35、55和60 ℃下进行厌氧消化实验发现高温能够很好地减少沙门氏菌及大肠杆菌等病原体的释放。FERNÁNDEZ-RODRIGUEZ et al[12]利用Romero模型对中高温厌氧消化微生物最大生长速率(mu(MAX))进行拟合,结果表明高温过程生长速率较中温过程提高27%~60%,并在更短时间内实现相同水平有机物的降解;同时WANG et al[13]指出通过改变温度进而改变水解酸化细菌和产甲烷菌相对丰度来提高厌氧消化工艺性能。但高温厌氧消化中的菌群易受外部环境的影响,其在厌氧消化过程中的耐热机理及微生物调节机制还有待进一步研究。所以本研究通过对比中高温厌氧消化产气特性及微生物群落结构变化,从分子生物学角度分析其各自微生物调节机制以及高温厌氧消化耐热机理,为实际沼气工程提供理论指导,实现能源高效回收。
-
原材料鸡粪取自沈阳市某养鸡场,接种污泥取自沈阳市北部污水处理厂污泥浓缩池,取回后利用鸡粪进行驯化处理,保持接种污泥中微生物活性,鸡粪与接种污泥特性,见表1。
-
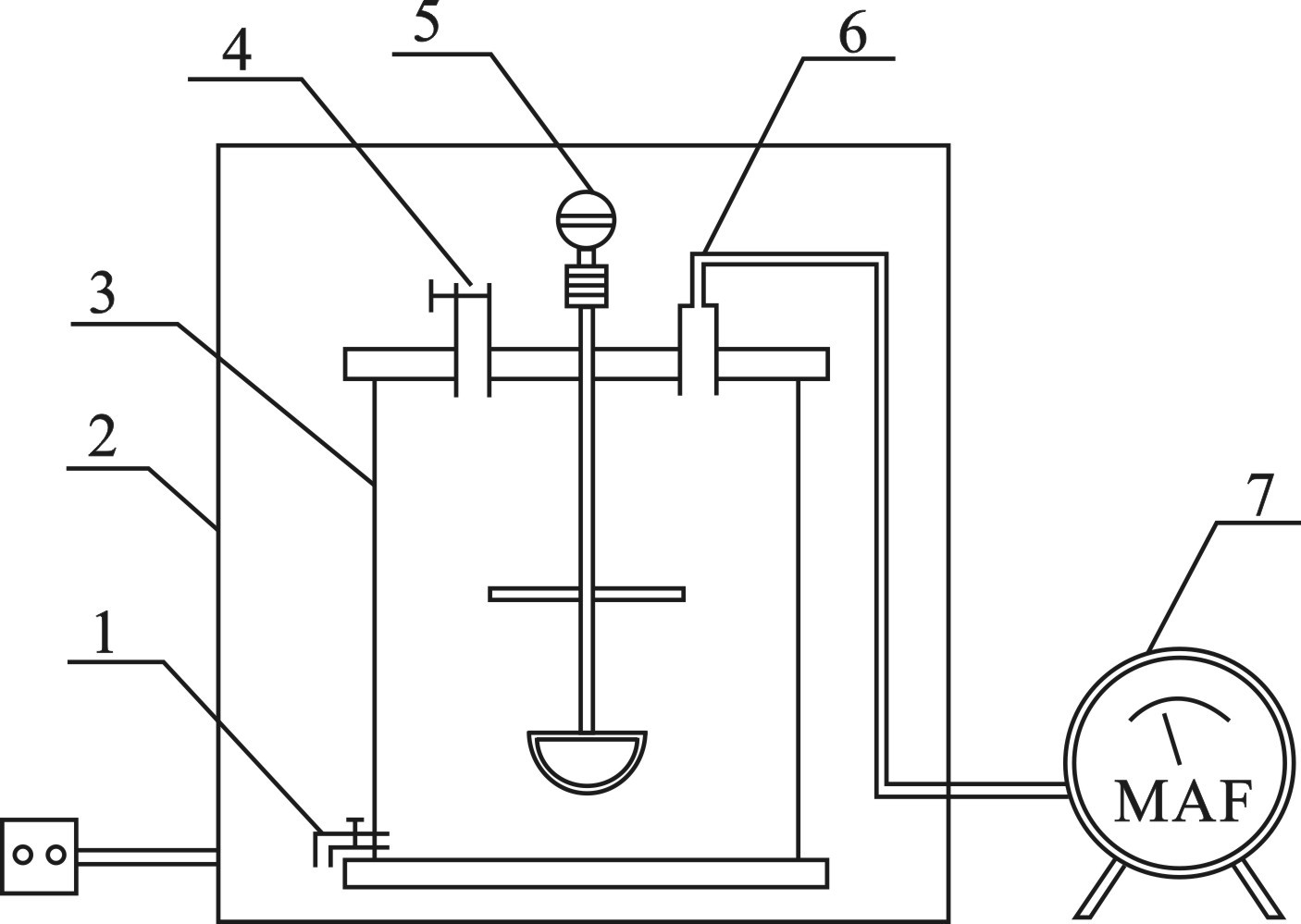
本实验采用自行设计的4个3 L独立厌氧消化反应器,见图1。
罐体容积为3 L,反应有效容积为2.5 L,反应器设置2组,每组反应器内一次性添加鸡粪150 g、接种污泥500 mL,后用去离子水定容至2.5 L,启动前通过向其通入氮气,保证其严格厌氧的环境。温度分别设置为37和55 ℃,每组实验设置一组平行实验,实验结果取其平均值。实验过程中每隔6 h搅拌一次,每次2 min,待测样品由取料口取出,产生沼气通过湿式气体流量计实时监测,并定期检测pH、SCOD、氨氮、VFAs、菌群多样性等参数变化。
-
沼气产量通过湿式气体流量计(LMF-1)测得,甲烷产量通过甲烷气体检测仪(JK60-CH4)测量并计算测得,物料初始TS和VS通过重量法(GB/T28731—2012)测得,溶解性化学需氧量(SCOD)采用重铬酸钾法进行测量(GB/T32208—2015),利用纳氏试剂分光光度法测量氨氮(TAN)(HJ535—2009),利用比色法测量总挥发性脂肪酸(VFAs)。
采用16S rRNA基因测序来分析微生物多样性,首先将样品中的DNA采用CATB方法进行提取[14],并用琼脂糖电泳检测DNA的纯度和浓度。选用特异引物分别对细菌和古菌进行PCR扩增。PCR产物用2%的琼脂糖凝胶进行电泳检测,后进行文库的构建和上机测序。并利用PICRCUt进行基因代谢功能预测,将本实验的基因测序数据与代谢功能已知的菌群全谱系基因功能预测谱数据库进行对比,从而实现对基因代谢功能的预测。其中中高温微生物群落样本分别用M(Medium)和H(High)表示,M1—M8与H1—H8分别代表中高温序批式厌氧消化实验随时间变化的8次测量结果。
-
温度是影响厌氧消化过程有机物降解效率的关键因素,中高温厌氧消化过程中甲烷产量、pH、SCOD、氨氮等常规参数的变化,见图2。
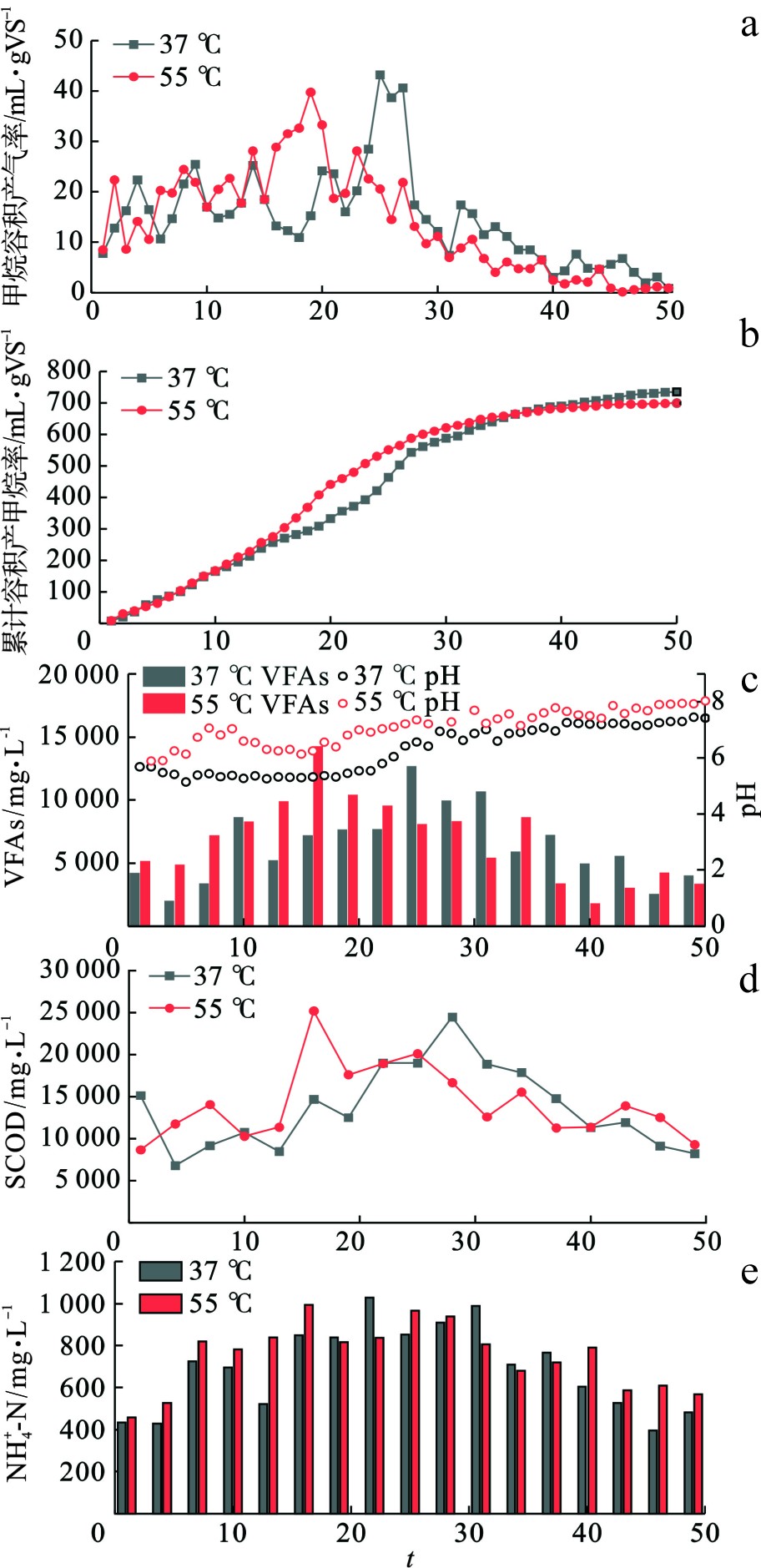
图2(a)可知,在实验前期底物基质相同的情况下,中高温甲烷日产量变化趋势大致相同,在实验中期高温组甲烷日产量于第19天率先达到峰值,产量为39.77 mL/gVS,中温组于第25 d达到峰值,产量为43.18 mL/gVS,结果表明高温对厌氧消化中有机物具有更高的降解效率[15],但倘若反应系统内有机质含量过高,高温厌氧消化内会出现较高的碱度和氨氮水平,从而对微生物活性产生抑制。图2(b)可知,中高温厌氧消化累计产甲烷量分别为734.75和699.50 mL/gVS,由于底物中有机质含量较高,首先在高温条件下嗜热菌群对温度具有较高耐受性展现出强大的活性,其他菌群在高温环境下会通过提高自身新陈代谢来提高自身耐受性,使系统产生更多的VFAs,从而影响有机质的降解效率[16];但与中温组相比,高温组累计产甲烷量曲线能够较快增长并趋于平缓,同样表明高温下有机物降解率更高,能够有效缩短厌氧消化反应周期。
厌氧消化系统内最适pH为6.8~7.2,过高或过低的pH均会对水解酸化细菌和产甲烷菌等微生物产生不同程度的抑制效果。图2(c)反映了pH和VFAs的变化关系,研究发现中高温实验组pH均呈现出前期较低对着反应进行逐渐升高的变化趋势,VFAs也整体呈现出先上升后下降的变化趋势。这是由于大分子有机物在水解酸化细菌的作用下降解为脂肪酸等小分子有机物,从而使VFAs含量逐步上升,其间同时产生大量游离H+导致pH下降。随着反应进行,产甲烷菌逐渐适应了环境并开始利用系统中游离的H+与VFAs合成CH4,VFAs含量开始下降,pH值升高,这与YANG et al[17]的研究结果相似。通过对比发现高温组在整个实验过程中的pH均高于中温组,且VFAs也率先达到峰值,原因是底物中含有较多粗蛋白,蛋白质在高温条件下的转化速率更快,从而提高了系统内的总碱度,对VFAs起到了一定的中和作用[8,18]。ALMEIDA et al[19]发现高温厌氧消化在不需要人为干预的情况下能够使pH稳定在7.0左右,而中温厌氧消化则需要加碱来维持反应系统pH的稳定。
SCOD的含量能够直观地反映出厌氧消化系统内有机质的含量,图2(d)可以看出SCOD随有机质水解呈现出先上升后下降的变化趋势,且高温组在第16 d率先达到峰值(25 170 mg/L),中温组在28 d达到峰值(24 470 mg/L),在序批式实验中底物浓度保持不变,SCOD浓度会随着产甲烷菌对有机质的利用而逐渐降低,产甲烷古菌OTU数目明显上升。在反应结束时,中高温SCOD去除率分别达到66%和63%,SCOD含量的变化能够从侧面反映出系统中的有机物得到有效降解[20]。DUAN et al[21]研究发现TAN浓度会对厌氧消化系统产生不同的抑制效果,当TAN浓度在0~3 000 mg/L时不会对系统产生抑制现象,高温产甲烷菌对氨氮的耐受性阈值为8 000 mg/L,中温产甲烷菌为16 000 mg/L,所以温度的升高会增加氨氮抑制的风险[22-23]。本研究中高温厌氧消化实验氨氮浓度变化如图2(e)所示,由于底物浓度较低,氨氮浓度均在3 000 mg/L以下,不会产生明显的氨抑制现象。由于高温厌氧消化比中温厌氧消化更易受到氨氮抑制,所以在实际工程中尤其在高温厌氧消化工艺中,应避免氨氮浓度过高,使厌氧微生物处于可承受范围之内,保障系统稳定运行。
-
本研究基于16S rRNA测序所得的有效数据进行OTUs物种聚类分析,分别得到1 717和1 941个细菌的OTU,中高温细菌样本群落变化,见图3。
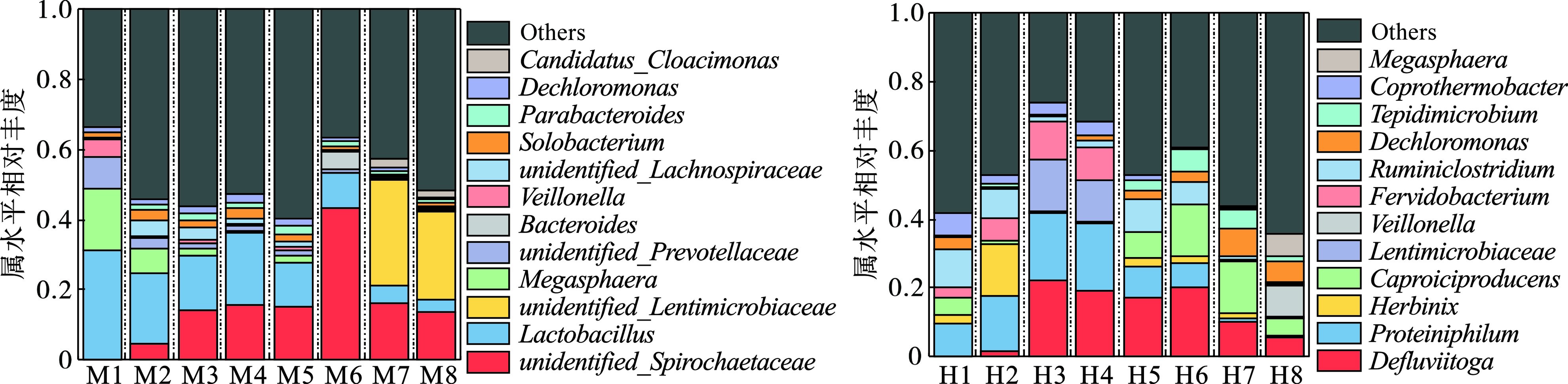
中温厌氧消化在门水平上优势菌种主要为Firmicutes、Spirochaetes、Bacteroidetes和Proteobacteria,相对丰度最高分别为69.11%、45.83%、36.30%、17.84%。这4种细菌门下的大部分微生物均可对有机质进行降解并转化为丙酮酸进而产生VFAs和乙醇等代谢产物[24]。Firmicutes中含有众多功能性微生物,在实验前期对大部分有机物(蛋白质、纤维素类、碳水化合物等)进行降解[25],随着有机质不断被消耗,菌群相对丰度逐渐降低。Bacteroidetes和Proteobacteria作为肠道菌群的优势菌种,主要作用于蛋白质,将其水解为氨基酸,并为自身及其他微生物提供维生素、乳酸和短链脂肪酸等营养物质[20,26]。高温厌氧消化在门水平上的优势菌种主要为Firmicutes、Thermotogae、Bacteroidetes和Proteobacteria,相对丰度最高分别可以达到72.61%、33.39%、35.15%、39.95%。其中Thermotogae是一类嗜热或超嗜热菌群,如热袍菌等细胞内具有错配修复机制(MMR),在受到热损伤后会使DNA片段发生碱基对错位配对,导致遗传信息发生改变,MMR会切除错误的碱基对,然后通过DNA聚合酶和连接酶的作用,合成配对正确的双链DNA分子。因此嗜热菌在DNA重组反应中发挥介导作用,细胞内部含有识别损伤、启动修复的重组介导功能的蛋白以提高细菌的耐热性。中温(左)和高温(右)温厌氧消化细菌菌群相对丰度柱形,见图4。
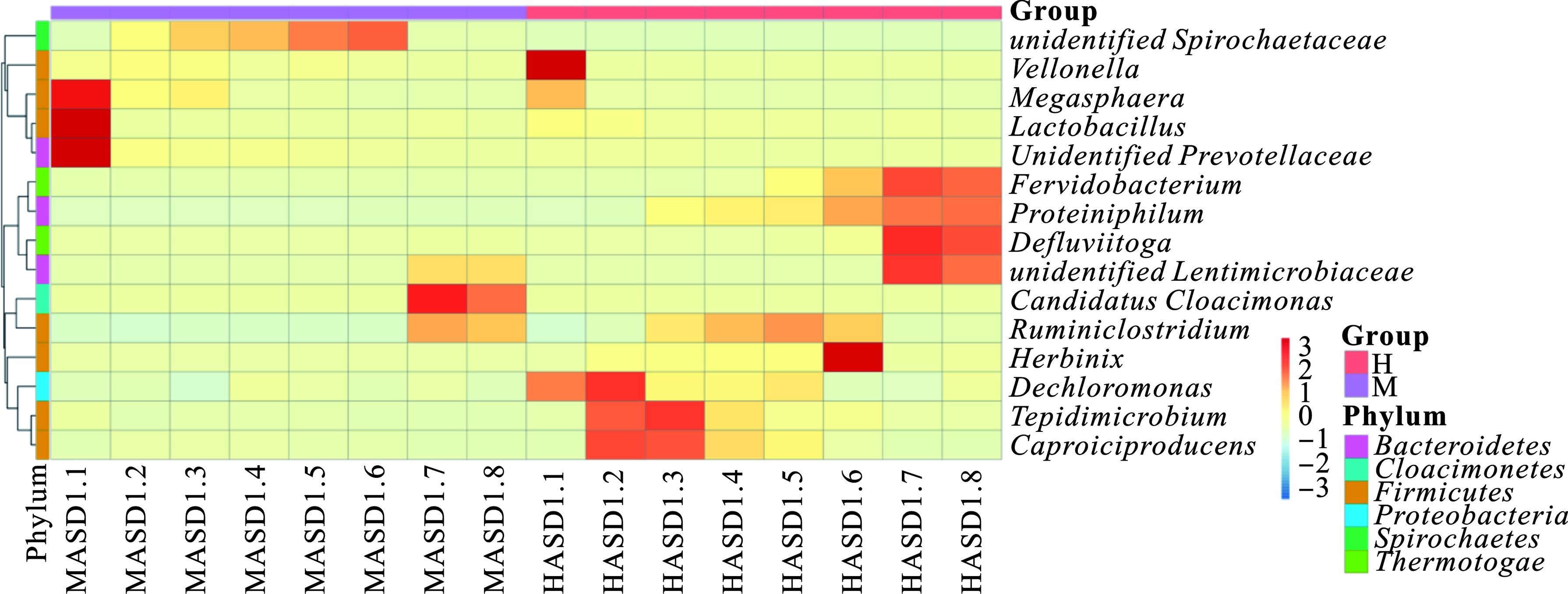
图4(左)可知,中温厌氧消化过程中属水平上优势菌属主要有unidentified_ Spirochaetaceae、Lactobacillus、Megasphaera和unidentified_Lentimicrobiaceae,其中unidentified_ Spirochaetaceae和Lactobacillus相对丰度最高可达43.08%和20.19%,unidentified_Spirochaetaceae属于螺旋体科(Spirochaetaceae),是一种常见的人畜共患病的病原体,大多存在于被污染的水体及其他环境中,可利用糖类、氨基酸及长链脂肪酸等以维持自身能量需求[27]。Lactobacillus隶属于乳杆菌科,可产生乳酸及多种脂肪酶,可将有机质主要转化为丁酸等脂肪酸类物质,通过PENG et al[28]研究发现其在酸性环境中仍然具有较高活性,能够为其他细菌提供可利用的有机硒等微量元素,这些微量元素在一定程度上可减缓细胞凋亡,但Lactobacillus与其他细菌的互营共生关系还有待进一步研究[29]。Lactobacillus随着反应进行丰度逐渐下降,这与底物浓度及反应系统内的pH变化有关。Megasphaera属于实验前期优势菌种,主要利用Lactobacillus产生的乳酸及果糖[30],其丰度会随着Lactobacillus变化而变化。unidentified_Lentimicrobiaceae是一种严格厌氧、短棒状革兰氏阴性细菌,最适pH为7.0左右,由于实验后期pH升高致使其丰度主要体现在中温厌氧消化后期;SUN et al[31]指出Lentimicrobiaceae主要作用于碳水化合物,发酵终产物是乙酸盐、苹果酸盐、丙酸盐、甲酸盐和氢离子。
高温厌氧消化属水平优势菌种主要为Defluviitoga、Proteiniphilum、Herbinix、Caproiciproducens等,相对丰度最高分别可达22.14%、16.68%、15.39%、15.34%。图4(右)可知,值得注意的是高温厌氧消化中发现的菌群对温度耐受性较高。Defluviitoga 作为高温厌氧消化优势菌种,属于Thermotogae门,是一种嗜热微嗜盐厌氧的化学有机营养型细菌,最适温度为55 ℃,最适pH为6.5~7.9,具有发酵广谱碳水化合物和酵母提取物的能力,可将大部分的多糖类物质(如葡萄糖、果糖、半乳糖、蔗糖等)用作电子供体,降解并转化为醋酸盐、H2和CO2,还可将硫代硫酸盐和元素硫还原为H2S[32-33]。Proteiniphilum是一种嗜蛋白质嗜纤维素的功能性细菌,最终产物为乙酸,其在高温厌氧消化反应前中期丰度较高,并随着反应进行丰度逐渐下降,这与系统内底物浓度变化有关。WU et al[34]检索并筛选了该属四个基因组是否存在碳水化合物活性酶,结果表明所有分析的基因组都包含多种参与逐步水解的酶,包括糖苷水解酶(GH)、碳水化合物酯酶(CE),并验证了其功能性。Herbinix和Caproiciproducens的最适温度范围为40~65 ℃,为高温厌氧消化过程中的次优势菌种,其中Herbinix主要作用于纤维素,将纤维二糖降解为乙酸、乙醇、丁酸和氢气等产物[35]。Caproiciproducens可将果糖作为底物,转化为乳酸、乙酸、正丁酸、正己酸、H2和CO2,ESQUIVEL-ELIZONDO et al[36]将其全基因组和反向β氧化基因与其他细菌进行了比较,同样验证了这一观点。
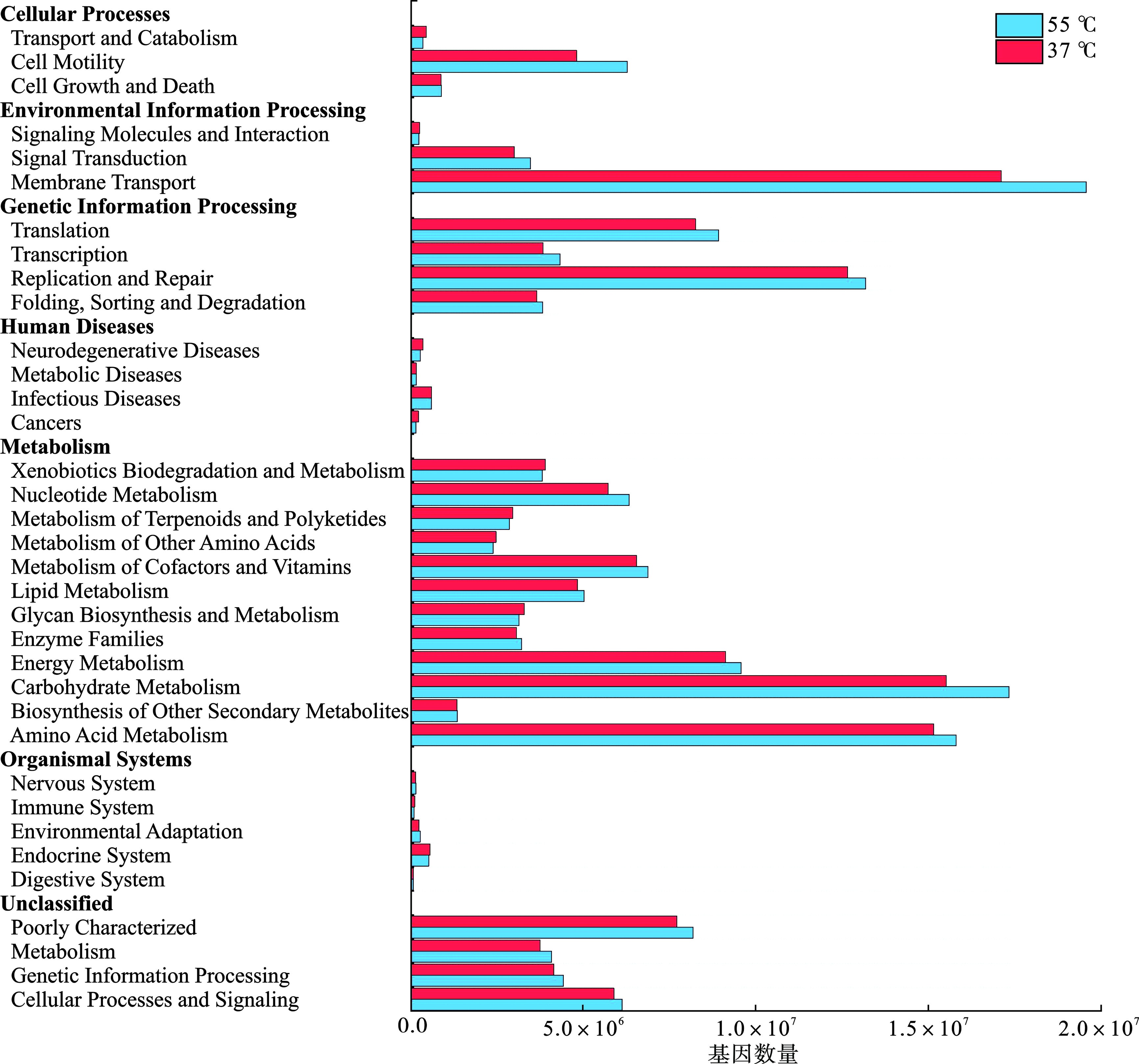
通过对中高温16S rRNA测序结果细菌群落基因功能预测,分析中高温细菌群落在基因功能上的差异,见图5。
细菌结构域中注释的序列主要分为6个功能组:细胞过程、环境信息处理、遗传信息处理、人类疾病、新陈代谢和有机系统,其中细胞之间的膜运输、遗传信息的复制与修复、碳水化合物和氨基酸的代谢所占基因数量最高,可作为细菌基因的主要功能表达。通过对比中高温细菌群落功能预测可以看出,高温厌氧消化细菌基因表达在6个功能组中均高于中温组。造成这种现象的原因是高温会促进细胞的新陈代谢,提高细胞与环境之间的物质交换效率,促进细胞内遗传信息的表达。TIAN et al[37]也指出随着温度的升高,水解酸化细菌代谢明显增多,且涉及水解、产酸阶段的细菌多样性明显增加,细菌在高温环境的刺激下,通过提高新陈代谢,促进肽链中二硫键的形成,增加双链DNA分子的空间位阻,来保证其在高温下的活性。
-
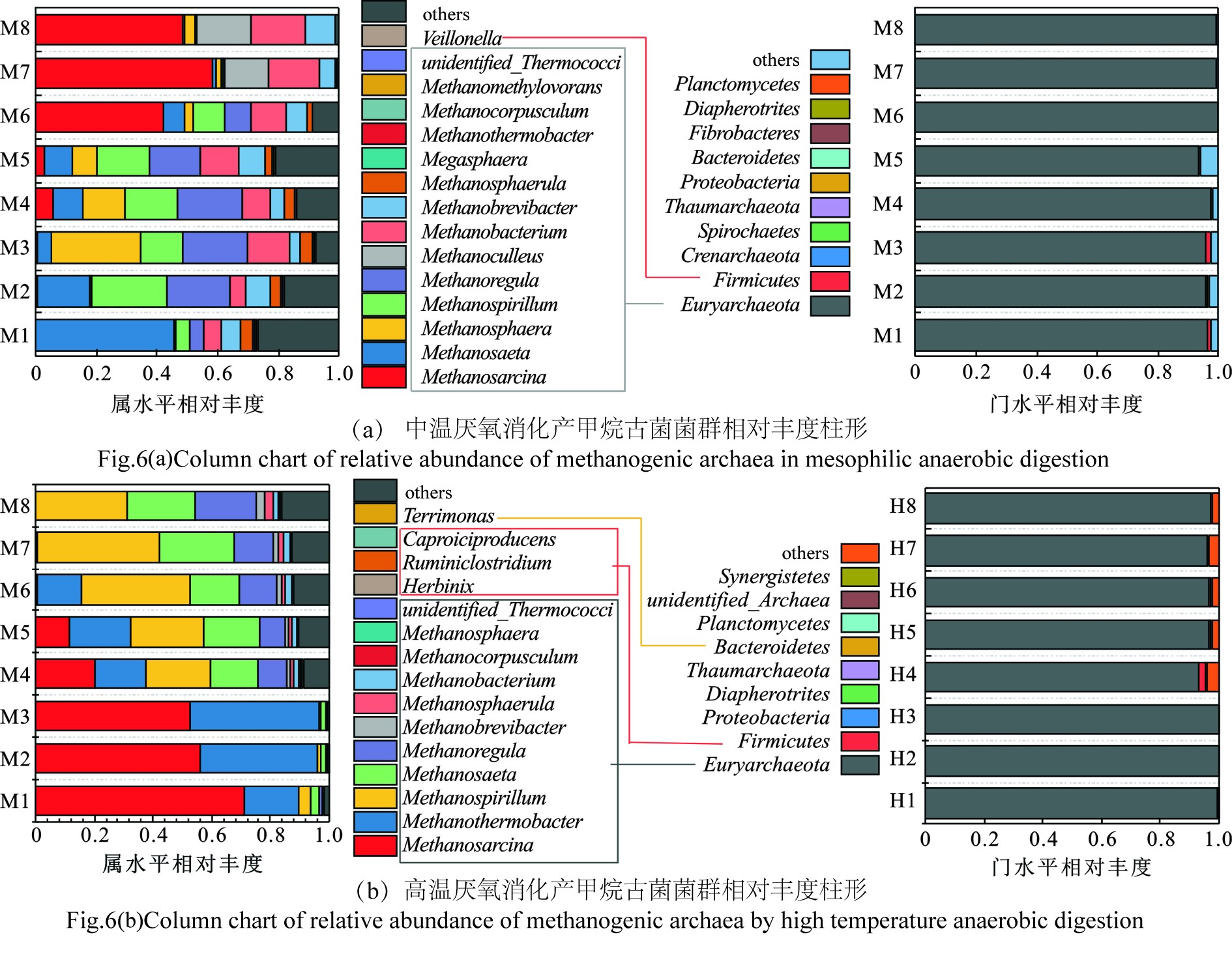
中高温厌氧消化古菌门水平优势菌种主要为Euryarchaeota,Euryarchaeota在16S rRNA物种进化树上属于一个单系群,包含了古菌中大部分群落,产甲烷阶段的产甲烷菌以及嗜热、嗜盐的厌氧菌等均来自Euryarchaeota门。中高温厌氧消化古菌菌群相对丰度,见图6。
图6(a)可知,中温厌氧消化属水平优势菌种主要为:Methanosarcina、Methanosaeta、Methanosphaera、Methanospirillum、Methanoregula,相对丰度最高分别可达到45.38%、24.73%、29.68%、21.50%和18.24%,其中Methanosaeta属于食乙酸产甲烷古菌,主要将乙酸和H2转化为CH4,在厌氧消化过程中,也可在一定程度上利用其他类型底物,例如将甲基胺或甲醇歧化为CH4和CO2[38]。在中温厌氧消化实验中由于底物逐渐被降解,其丰度随着反应进行逐渐降低,符合微生物生态学变化规律。Methanosarcina是已知的唯一一种可利用所有甲烷代谢途径的菌群,最适pH为6.5~8,由于受pH影响,主要作为在实验后期优势菌种,Methanosarcina的第一种代谢途径为利用乙酸,并转化为H2和CO2,然后将H2和CO2作为原料合成CH4;第二种代谢途径是在甲基转移酶的作用下将甲醇、甲胺等化合物转化成甲烷[39]。值得注意的是,Methanosarcina是厌氧消化过程中最重要的产甲烷菌群,还可根据环境温度划分为嗜温甲烷八叠球菌和嗜热甲烷八叠球菌[39]。
高温厌氧消化产甲烷古菌属水平优势菌种相对丰度如图6(b),可以看出其在属水平上的优势菌种主要为Methanosarcina、Methanothermobacter、Methanospirillum、Methanosaeta和Methanoregula,由于高温厌氧消化pH一直处于6.5以上,所以Methanosarcina在实验前期即表现出较大的优势,并且丰度随着有机底物消耗而逐渐下降。Methanothermobacter是一种热自养氢营养型甲烷嗜热杆菌,最适生长温度为65~70 ℃,为实验前期优势菌种,可以将H2、甲醇等物质转化为CH4和CO2,以提供自身细胞生理活动需求[40],麻婷婷等[41]同样发现Methanothermobacter是高温条件下石油烃降解产甲烷的优势古菌之一。在实验后期优势菌种主要为Methanospirillum和Methanosaeta,通过对比可以看出中温厌氧消化大部分产甲烷优势菌种同样出现在高温厌氧消化过程中,造成这种现象的原因是这些产甲烷菌对高温具有较好的耐受性,细胞中的DNA在高温条件下触发损伤诱导反应(DDR),激活修复蛋白,修复DNA损伤,维护细菌基因组的稳定性[42]。SUTER[43]指出DDR是多层次、多水平调控,与损伤信号转导和DNA修复有关的基因多达500多个;当DNA受到外界环境胁迫造成损伤时MRN (X)、ATRIP、Rad17等损伤识别蛋白会通过ATM、ATR等蛋白酶将信号传递给效应蛋白,进而影响DNA合成、细胞周期、细胞凋亡、衰老以及DNA修复等细胞进程[44]。中高温古菌群落功能预测,见图7。
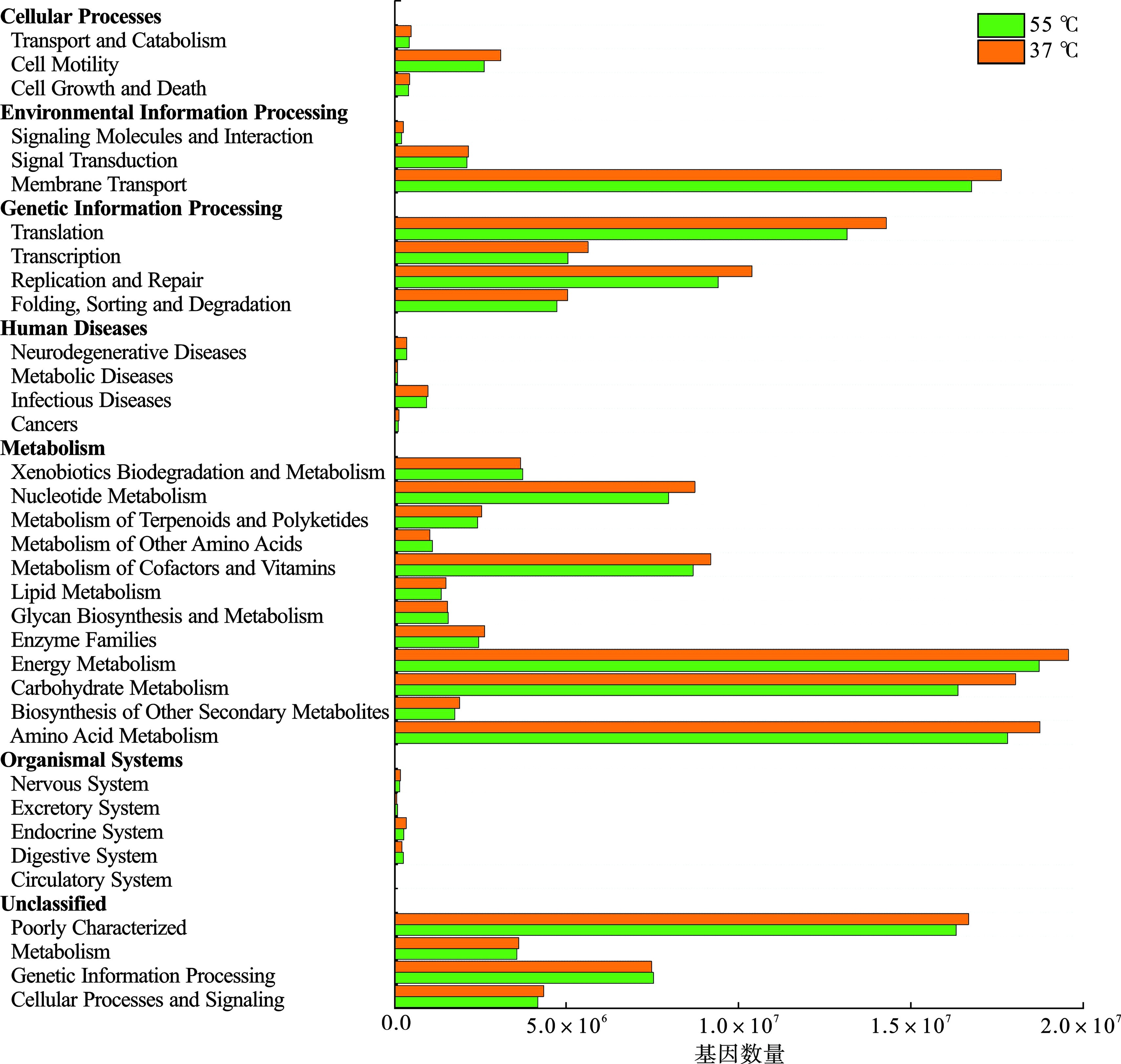
通过利用16S rRNA基因测序数据与代谢功能已知的微生物基因组数据库进行对比,实现了中高温产甲烷古菌基因的功能预测,见图7,古菌结构域中注释的序列同样分为六个功能组:细胞过程、环境信息处理、遗传信息处理、人类疾病、新陈代谢和有机系统,其代谢通路主要为能量代谢、碳水化合物代谢、氨基酸代谢、膜运输、遗传信息翻译与表达,KUNDU et al[45]在中高温连续式厌氧消化实验中发现,与在55 ℃下运行的反应器相比,37 ℃下的反应器能够承受更高的有机负荷且微生物群落更加多样化,这与本实验研究一致。中温厌氧消化过程中基因的代谢通路均高于高温厌氧消化,造成这种现象的原因是高温阻断了部分乙酸营养型产甲烷菌的代谢通路,主要以氢营养型产甲烷代谢通路为主,并得到了其他属明确定义的共生产氢细菌的支持,这与GAO et al[46]研究结果一致。
-
为了进一步探究微生物群落结构,基于16S rRNA数据集对中高温厌氧消化α多样性指数进行评估[47],细菌和古菌的α多样性见表2与表3,本研究每组样品生物覆盖率均在0.99以上,可以认为测序深度已基本覆盖样品中的所有物种。
表2可知,高温厌氧消化过程中样品observed species指数较高,所含物种较为丰富,且其自身丰富度变化与厌氧消化系统内的底物浓度呈正相关变化,与WITTEBOLLE的研究结果一致[48]。Shannon和Simpson能够评估物种的均匀度,值越大表示各物种分配越均匀[49-50],可用来评估优势菌群在反应系统中的地位和作用,可以看出中高温各组样品均匀度指数相差不大,物种分配较为均匀。Chao1和ACE的作用与物种数指数类似,数值越大表示表示物种丰富度和多样性越高[51],通过对比中高温多样性指数发现,在细菌域中高温厌氧消化的Chao1和ACE指数整体高于中温厌氧消化,而在古菌域中高温厌氧消化的Chao1和ACE指数整体较低,造成这种现象的原因是水解酸化细菌所覆盖的菌群数量远比产甲烷古菌多,样本中的OUT数目即可验证这一观点,温度的变化使水解酸化阶段衍生出更多细菌,产甲烷古菌在受到温度的影响后,在底物充足的情况下,尚且可以通过调节自身代谢来适应环境胁迫,但随着底物浓度不断被消耗,没有足够能量来维持自身生理活动,多样性指数开始下降,这与前文的研究可以形成呼应。
-
(1)高温厌氧消化比中温厌氧消化提前6 d达到产甲烷峰值,由于底物浓度相同,中高温累计产甲烷量相差不大,高温组pH始终高于中温组,能够在更短时间达到相对稳定的pH,系统不易产生酸化现象,且VFAs与SCOD的变化与甲烷产量变化相似,说明高温条件更有利于有机物的水解酸化和甲烷的生成。
(2)中温厌氧消化水解酸化阶段优势菌种为unidentified_ Spirochaetaceae、Lactobacillus和Megasphaera,对酸性环境具有较好的抗逆性,产物为乙酸和丁酸等脂肪酸类物质;高温水解阶段的优势菌种主要为Defluviitoga、Proteiniphilum、Herbinix和Caproiciproducens等,其对高温环境具有较好的抗逆性,其中Defluviitoga可将大部分多糖类物质当做电子受体,并降解为醋酸盐、H2和CO2,提高了水解酸化效率,使厌氧消化反应周期缩短12%,同时高温环境减少了病原体的产生。中高温厌氧消化产甲烷优势菌群也存在较大差异,且分别属于不同的功能性产甲烷菌。
中高温鸡粪厌氧消化微生物调节机制对比及耐热机理研究
Comparison of microbial regulation mechanisms and heat-resistant mechanism of anaerobic digestion of chicken manure at medium and high temperature
-
摘要: 温度对厌氧消化系统内微生物调节机制的影响尚不清楚,通过对比中高温厌氧消化实验,分析了其各自微生物调节机制以及高温厌氧消化耐热机理。结果表明,在底物浓度相同的情况下高温厌氧消化比中温提前6天达到产甲烷峰值,高温条件能够缩短厌氧消化反应周期,更有利于有机物的水解酸化和甲烷的生成;Defluviitoga作为高温厌氧消化过程水解阶段优势菌种,对高温环境具有较好的抗逆性,可将大部分多糖类物质当作电子受体,并降解为醋酸盐、H2和CO2;Methanosarcina作为中高温厌氧消化产甲烷阶段优势菌种,能够适应中温高温两种不同环境,且可利用所有甲烷代谢途径(食乙酸、食氢、食甲基化合物),产甲烷潜力巨大。Abstract: The influence of temperature on the microbial regulation mechanism in the anaerobic digestion system is unclear. The microbial regulation mechanisms and the mechanism of heat resistance of high-temperature anaerobic digestion by comparing the anaerobic digestion experiments at medium and high temperatures were analyzed. The results showed that under the condition of the same substrate concentration, high-temperature anaerobic digestion reached the peak of methane production 6 days earlier than medium-temperature, and high-temperature conditions could shorten the reaction cycle, which was more conducive to the hydrolysis and acidification of organic matter and the formation of methane. Defluviitoga, as the dominant strain in the hydrolysis stage of the high-temperature anaerobic digestion process, had good stress resistance to high-temperature and could use most polysaccharides as electron acceptors and degrade them into acetate, H2 and CO2. Methanosarcina, as the dominant strain in the methanogenic stage of medium-high temperature anaerobic digestion, could adapt to two different environments, use all methane metabolic pathways (eating acetic acid, hydrogen, and methyl compounds), and has great potential for methane production.
-
厌氧发酵是一种能够有效实现有机废物资源化和能源化的生物反应过程[1]。在我国,餐厨垃圾(FW)每年的产生量约为6×107 t,占城市固体废弃物总量的40%以上[2]。FW主要由易于降解的碳水化合物、蛋白质和脂质组成,具有较高的产甲烷潜力[3-4]。但是,单独发酵FW时,由于FW水解速度较快会积累挥发性脂肪酸(VFA),易发生系统抑制崩溃的后果[5]。已经有研究证明将剩余活性污泥(WAS)添加到FW厌氧发酵系统提高混合发酵运行性能的可行性[6]。与单独FW或WAS厌氧发酵相比,将2者进行厌氧混合发酵能够促使微生物发挥协同作用,稳定厌氧发酵性能。
目前,有关FW和WAS厌氧混合发酵系统的构型主要采用间歇进料的连续搅拌反应器(CSTR)[7-8]。然而,CSTR不能实现污泥停留时间(SRT)和水力停留时间(HRT)的有效分离,使得微生物难以持留,难以保障微生物的持续生长,而且CSTR的间歇式进料方式容易引起负荷冲击。动态膜生物反应器(DMBR)使用在膜基材表面上沉积/吸附形成的滤饼层作为过滤层,能有效防止生长缓慢的厌氧微生物尤其是产甲烷菌的流失,提供了较长SRT来维持大量微生物种群生长[9]。已有研究利用板框内置式膜组件,采用连续流运行模式,在2.8 g·L−1·d−1的负荷下,实现了基于DMBR进行玉米秸秆和FW的混合发酵[10]。连续流进料方式可以有效缓解间歇式进料方式引起的基质冲击,增加系统的缓冲能力。目前,有关连续流动态膜厌氧混合发酵系统的稳定运行的解析鲜见报道。
在厌氧混合发酵系统中,基质的混合比例是影响厌氧发酵的关键参数,李浩等[11]的研究结果表明,在FW和WAS厌氧混合发酵过程中,FW所占比例影响混合发酵的反应速率。同时,厌氧发酵系统的最优基质混合比也会随着系统的长期运行和菌群结构的驯化改变而变化[12]。食微比(F/M)是衡量有机负荷的重要参数[13],F/M与基质种类和接种物中微生物菌群密切相关,不同的F/M会影响系统的效能潜力。截至目前,很少有研究考虑基质混合比(FW/WAS)和F/M对厌氧混合发酵系统长期运行的影响。
本研究构建了FW和WAS的外置式动态膜厌氧混合发酵系统。在连续流条件下启动动态膜厌氧混合发酵系统,以实现系统的稳定运行;同时,对DMBR运行过程中动态膜的形成和固液分离的效果进行解析。通过FW/WAS的产甲烷潜能和动力学实验,优化连续流厌氧混合发酵系统的因素,结合F/M 动力学实验,评价FW/WAS与F/M对连续流厌氧混合发酵系统运行效能的影响。
1. 材料与方法
1.1 实验装置
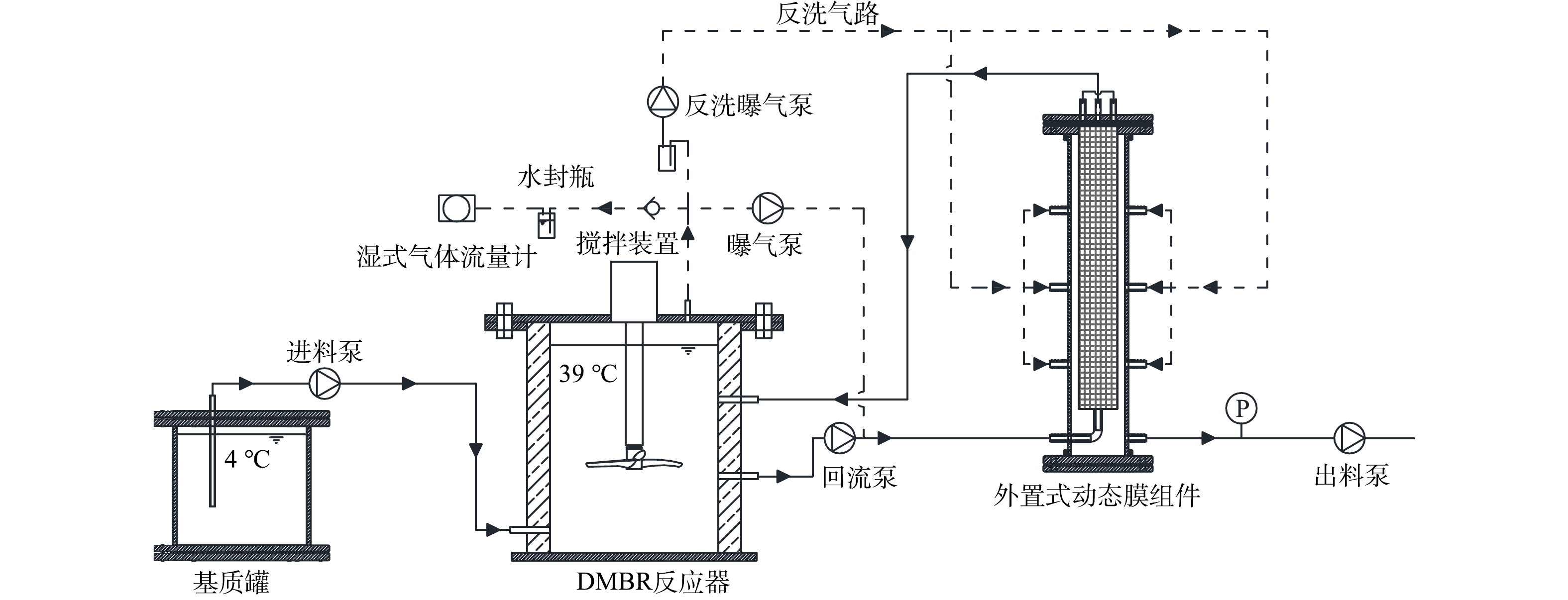
本研究使用的外置式动态膜生物反应器如图1所示。反应器的有效体积为9.0 L,外部使用水浴层和恒温槽来控制反应器的温度为 (39±1) ℃,基质罐连接4 ℃恒温冷水浴。外置式膜组件由300目不锈钢筛网定制加工而成,平均孔径为48 µm,有效过滤面积为0.047 m2。系统的运行模式为连续进出料,产生的生物气通过水封瓶后用湿式气体流量计计量产气量。通过曝气泵将系统内顶空生物气泵入膜组件腔体底部,对膜组件进行气擦洗后回流至系统内;同时,通过反洗曝气泵将系统内顶空生物气定期泵入膜组件腔体外侧,对膜组件进行气反洗后回流至系统内。当膜组件和出料泵间跨膜压差增加到40 kPa时,开启反洗曝气泵进行气反洗,反洗强度为10 L·min−1,气反洗时间为10 min。当进行气反洗不能提高膜通量时,通过增大曝气泵流量、回流量或气反洗频率进行调控。
1.2 基质和接种污泥
本研究所采用的FW依据学生食堂餐厨剩余物的主要成分进行人工模拟配制[14],WAS取自西安市第五污水处理厂,2者混合后添加微量元素作为最终混合基质[8]。启动阶段FW和WAS的混合比例为4∶1(基于湿重),该最优混合基质比是启动前期批次实验优化的结果[15]。研究所用接种污泥为FW和WAS中温厌氧CSTR的排泥[15],接种体积为9.0 L。本研究中使用的FW、WAS、混合基质和接种污泥的理化特性如表1所示。
表 1 基质和接种污泥的理化特性Table 1. Physicochemical properties of substrate and seed sludge供试对象 TS/(g·L−1) VS/(g·L−1) TCOD/(g·L−1) SCOD/(g·L−1) pH 乙酸/(g·L−1) 蛋白质/(g·L−1) 多糖/(g·L−1) NH4+-N/(g·L−1) FW 140.0±15.3 134.0±13.2 220.0±18.5 104.0±8.3 4.4 1.730 2.74±0.03 85.30±4.10 0.31±0.01 WAS 56.0±8.3 30.4±4.2 52.2±7.3 — — — — — — 混合基质 124.0±0.6 115.0±0.5 181.0±2.3 74.5±1.4 3.9 0.001±0.000 8.20±0.12 2.71±0.03 0.10±0.01 接种污泥 39.1±0.6 19.7±1.5 27.2±0.3 3.1±0.0 7.9 0.003±0.000 0.81±0.03 0.27±0.02 2.62±0.17 注:“—”表示未测定。 1.3 实验设置
设置DMBR系统的初始OLR和HRT分别为(1.84±0.45) g·L−1·d−1和62.5 d,启动运行72 d,测定系统的运行性能参数和动态膜截留性能。启动阶段运行结束后,采用批次实验进行FW/WAS和F/M参数优化,实验设置见表2。FW/WAS批次实验在F/M为0.145 (基于VS)时共设置7组,其中2组为FW和WAS单发酵。F/M批次实验在FW/WAS为4.4∶1时共设置8组。所有批次实验均在120 mL血清瓶中分批进行,同时设置空白组。其中,空白组与实验组均设置2组平行。当混合基质和接种污泥加入血清瓶摇晃均匀后,用氮气吹脱约3 min,橡皮塞封瓶后置于39 ℃恒温摇床内,摇床转速为120 r·min−1,2 min后血清瓶顶空放气,定时测定气组和气量。
表 2 批次实验的运行设置Table 2. Operating characteristics of the batch experiments实验项目 FW/WAS F/M 接种物/mL FW/mL WAS/mL 混合基质/mL 蒸馏水/mL FW单发酵 1∶0 0.206 30 0.905 0 — 3.095 WAS单发酵 0∶1 0.206 30 0 4.000 0 FW/WAS混合发酵 3∶1 0.206 30 0.680 1.000 — 2.320 FW/WAS混合发酵 4∶1 0.206 30 0.725 0.800 2.475 FW/WAS混合发酵 4.4∶1 0.206 30 0.740 0.740 2.520 FW/WAS混合发酵 5∶1 0.206 30 0.755 0.670 2.575 FW/WAS混合发酵 6∶1 0.206 30 0.775 0.575 2.650 F/M混合发酵 4.4∶1 0.090 30 — 0.960 14.040 F/M混合发酵 4.4∶1 0.176 30 1.865 13.135 F/M混合发酵 4.4∶1 0.354 30 3.750 11.250 F/M混合发酵 4.4∶14.4∶1 0.4720.567 3030 5.0006.000 10.0009.000 F/M混合发酵 F/M混合发酵 4.4∶1 0.708 30 7.500 7.500 F/M混合发酵 4.4∶1 0.944 30 10.000 5.000 F/M混合发酵 4.4∶1 1.417 30 15.000 0 注:“—”表示不适用。 1.4 测定项目和方法
TS、VS、COD、碱度和NH4+-N的测定采用标准方法[16]。pH采用便携式pH计进行测定(pHS-25型,上海精密科学仪器有限公司)。蛋白质和多糖分别采用Folin-酚试剂法[17]和硫酸-蒽酮法[18]。CH4、CO2、N2、H2和VFA均采用气相色谱法进行测定[8]。浊度采用便携式浊度仪 (Turb®355 IR,德国赛莱默公司) 测定。采用修正的Gompertz方程 (公式1) 拟合批次实验数据,以确定产甲烷潜力、最大产甲烷速率和延滞期[19-20]。采用一级动力学模型 (公式2) 进行数据拟合可得水解常数[21]。
P=P0⋅exp{−exp[Rmax⋅e⋅(t0−t)/P0+1] (1) P=P0⋅[1−exp(−kt)] (2) 式中:P为生物气产量,mL;P0为生物气潜能,mL;Rmax为最大生物气产生速率,mL·d−1;t0为延滞期,d;k为产甲烷速率常数,d−1。
2. 结果与讨论
2.1 反应装置的启动及运行性能
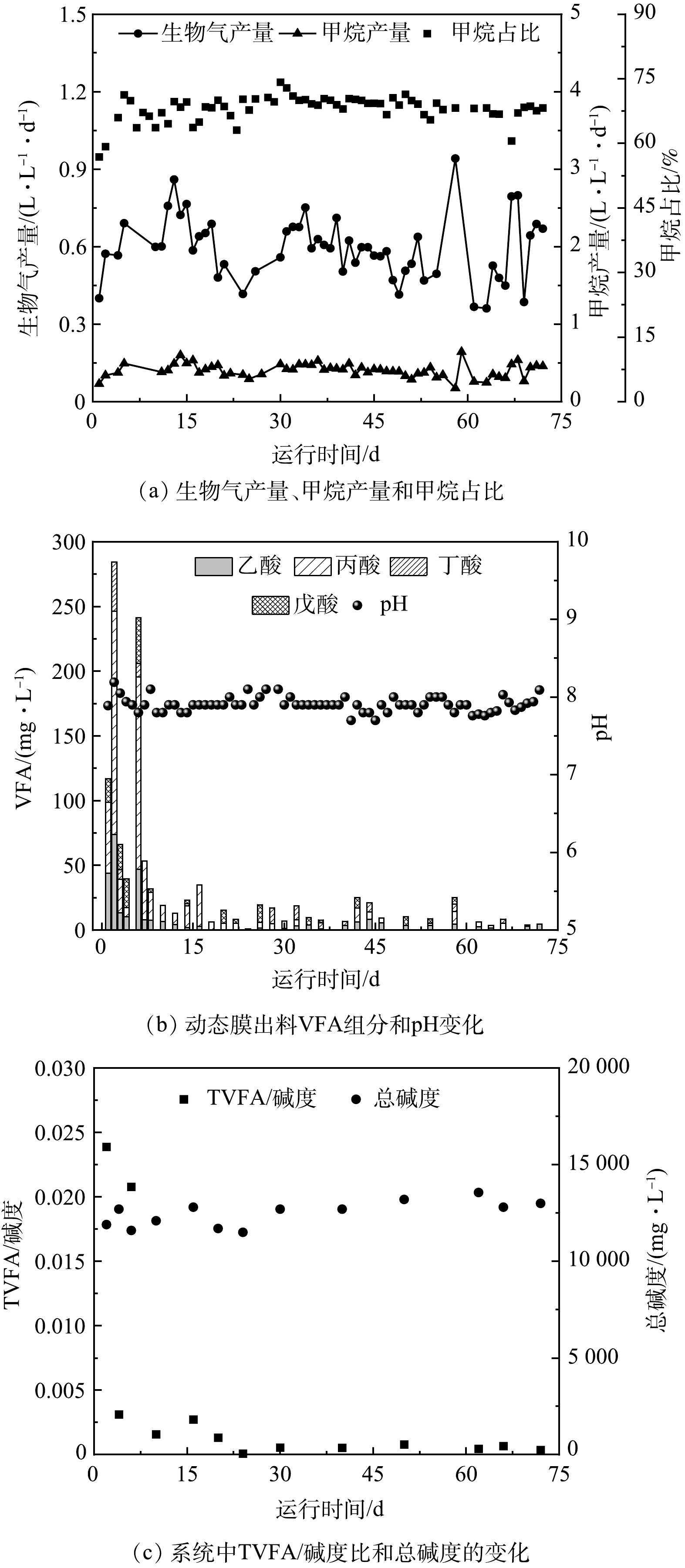
在HRT和OLR分别为62.5 d和(1.84±0.45) g·L−1·d−1的初始条件下,启动连续流FW和WAS厌氧混合发酵动态膜生物反应器。反应器启动运行过程中,系统的生物气产量、甲烷产量和甲烷占比如图2(a)所示。前5 d启动过程中,系统的生物气产量、甲烷产量和甲烷占比逐渐增加,然后趋于稳定。72 d的运行过程中,系统的平均生物气产量达到(0.60±0.11) L·L−1·d−1,平均甲烷产量达到(0.41±0.08) L·L−1·d−1,甲烷占比稳定在66%~71%,平均甲烷占比达到69.00%。pH和VFA的变化趋势能够直观的表明反应器的运行状况。如图2(b)所示,启动过程中,系统的pH始终稳定在7.6~8.0,在产甲烷菌最适pH(7.0~8.0)内[8]。本研究VFA最大质量浓度仅为284 mg·L−1,无VFA积累现象。这表明,连续流动态膜混合发酵系统启动成功[22]。如图2(c)所示,TVFA/碱度最大值仅为0.024,低于阈值0.4[23]。VFA和TVFA/碱度均未超过阈值,这表明厌氧发酵系统稳定性良好。厌氧发酵系统成功启动后,系统的平均TVFA质量浓度为(15.9±1.89) mg·L−1,低于产甲烷菌TVFA的抑制浓度5 000 mg·L−1,相应的总碱度为11 000~14 000 mg·L−1,也在稳定运行范围内[24]。上述结果表明,连续流FW和WAS厌氧混合发酵DMBR启动成功且能稳定运行。此外,对系统进行物料平衡分析可知,在该系统基质VSS的生物降解转化去除率为84%±3.8%,去除单位质量COD的基质甲烷产量为(294±13) mL。
2.2 动态膜的截留性能
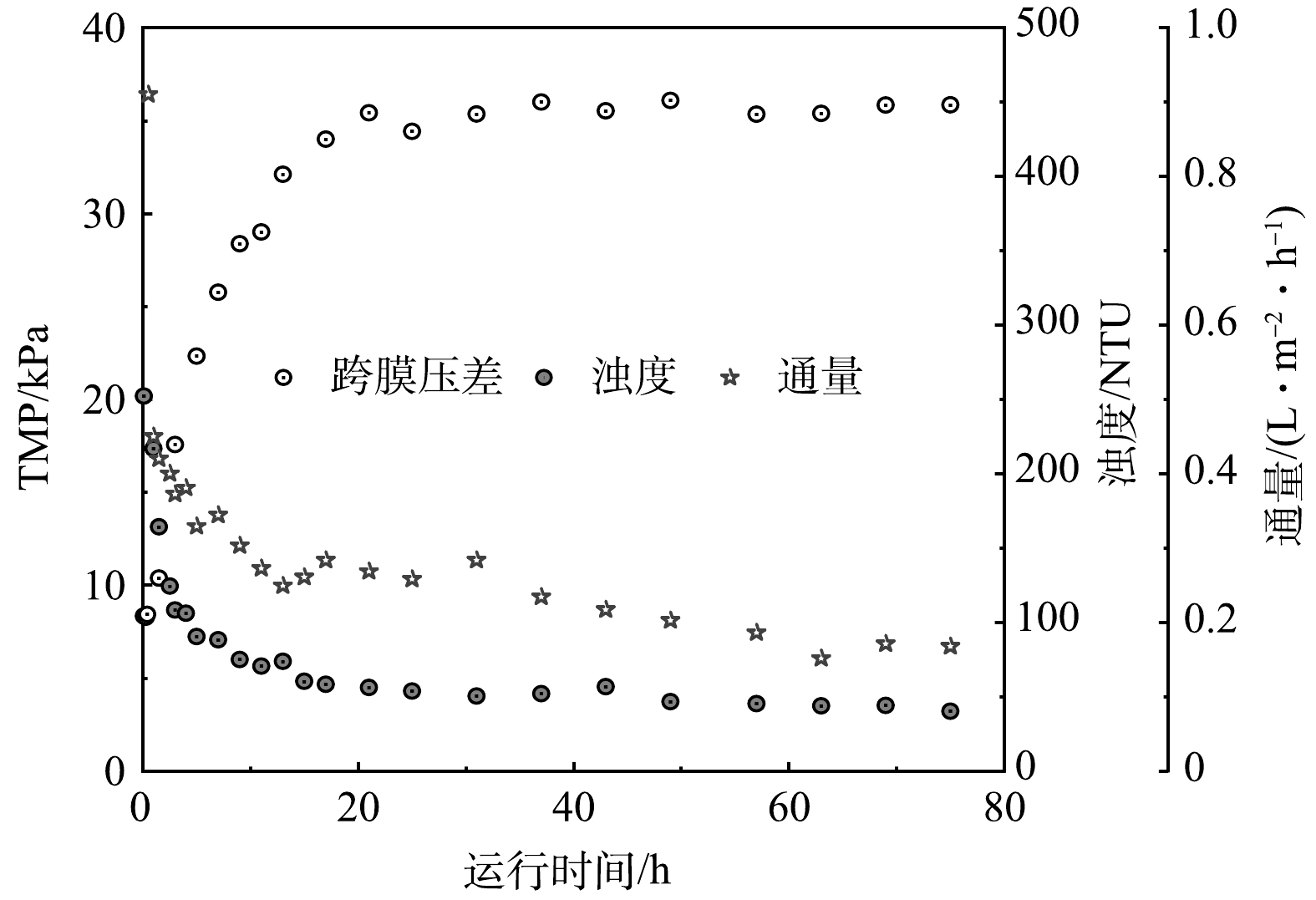
本实验的反应器装置为外置式的柱型动态膜组件,开启出料泵后,反应器内污泥先通过回流泵进入膜组件腔体内部,当回流污泥充满膜组件内部腔体后附着在动态膜基材上,逐渐形成过滤层。在第35 d膜组件清洗后,动态膜组件的跨膜压差、膜通量和浊度变化如图3所示。前4 h,动态膜组件的跨膜压差快速升高,由8.34 kPa增至22.3 kPa,相应的出料浊度由252 NTU降低至90.4 NTU,通量降低至0.42 L·m−2·h−1,2者均呈现快速下降的趋势。这是因为,动态膜组件腔体内充满了污泥,污泥开始附着在动态膜基材上,具有一定的截留效果。从4 h至21 h,通量降低了约40% (由0.42 L·m−2·h−1降至0.25 L·m−2·h−1) ,浊度也降至100 NTU以下,表明动态膜逐渐形成。随着过滤过程的进行,通量下降速度减缓,出料浊度趋于稳定。约40 h后,出料浊度稳定在50 NTU,通量在0.2 L·m−2·h−1左右。动态膜层逐渐增厚,进入稳定过滤阶段,具有稳定的截留效果。此外,当跨膜压差增至40 kPa时,进行动态膜气反洗后,能够快速形成动态膜,相应的压差逐渐增加 (如图3),长期运行过程中动态膜跨膜压差呈现周期性变化。袁宏林等[10]采用相同材质和孔径的动态膜基材,以玉米秸秆和FW为混合基质进行厌氧混合发酵,也获得了较优的固液分离效果,相应的有机物截留率达到95.9%,与本研究动态膜截留效果相当。通过借用在大孔径膜基材上形成的滤饼层作为过滤层,能够将传统膜生物反应器运行中存在的“膜污染”瓶颈问题转化为过滤层加以利用。本研究虽然对动态膜的过滤周期进行了表征,但仍需进一步解析动态膜滤饼层的过滤机理。此外,对接种物、运行末期动态膜滤饼层和系统排泥进行宏全基因组菌群分析可知:混合发酵系统以细菌为主,其中细菌主要包括Bacteroidetes (30.5%~44.6%) 、Chloroflexi (10.5%~24.5%) 和Firmicutes (23.1%~36.5%) ,古菌主要包括Methanosarcina (53.0%~97.9%) 和Methanobacterium (0.16%~18.7%) 。不同的微生物菌群结构组成及其变化,对于动态膜的形成和过滤效能均有一定程度的影响,但其作用机理仍需进一步研究。
为进一步揭示动态膜过滤截留效能的周期稳定性,在反应器运行的第7、15、21、28、41、53和60 d取样分析动态膜过滤液中TCOD、蛋白质及多糖质量浓度。如图4(a)所示,出料TCOD均低于3 g·L−1,且动态膜对TCOD的截留率可达到99.5%,最终可稳定在99%以上。这表明,该外置式动态膜组件可实现较好的出料质量,实现有机物和微生物的稳定截留。如图4(b)所示,经过动态膜出料的蛋白质和多糖质量浓度均低于300 mg·L−1,相应的蛋白质和多糖截留率均不低于95%。其中,出料蛋白质质量浓度始终高于多糖,主要由于混合基质中蛋白质质量浓度是多糖质量浓度的3倍以上 (表1) ;同时,出料蛋白质质量浓度逐渐下降,相应的去除率逐渐增加。分析其原因主要是,由于形成的动态膜对蛋白质的截留效果逐渐增强;相反,出料多糖质量浓度略有增加,相应的多糖截留率略有降低,但仍维持较高水平 (>95%) ,也与动态膜的过滤效能密切相关。动态膜滤饼层中蛋白质和多糖以及凝胶层对混合发酵系统中物质的截留作用是目前膜生物反应器探究的热点,相应的过滤截留机理有待进一步深入解析,以实现动态膜对蛋白质和多糖的截留调控。
2.3 运行参数的优化调控
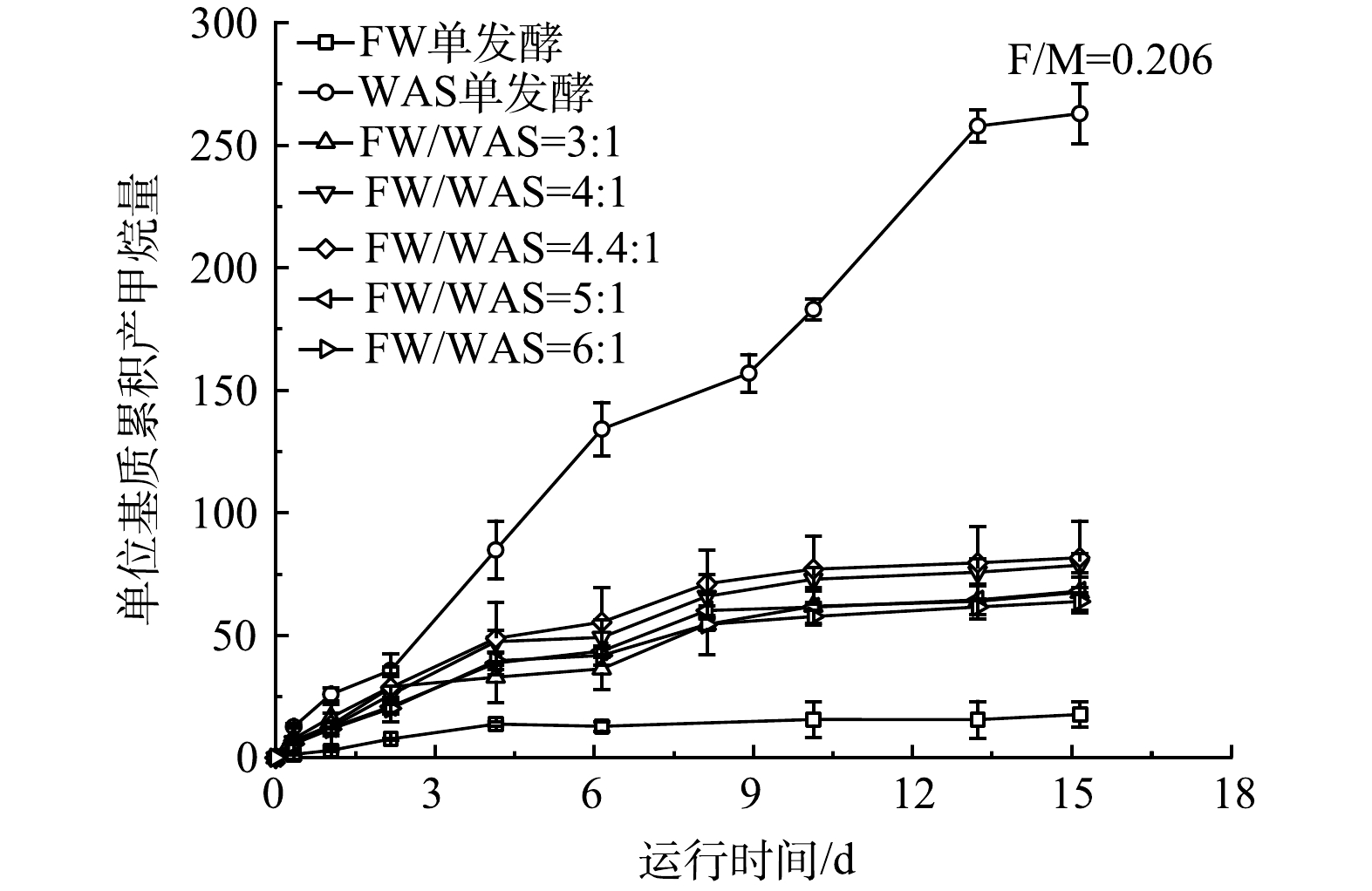
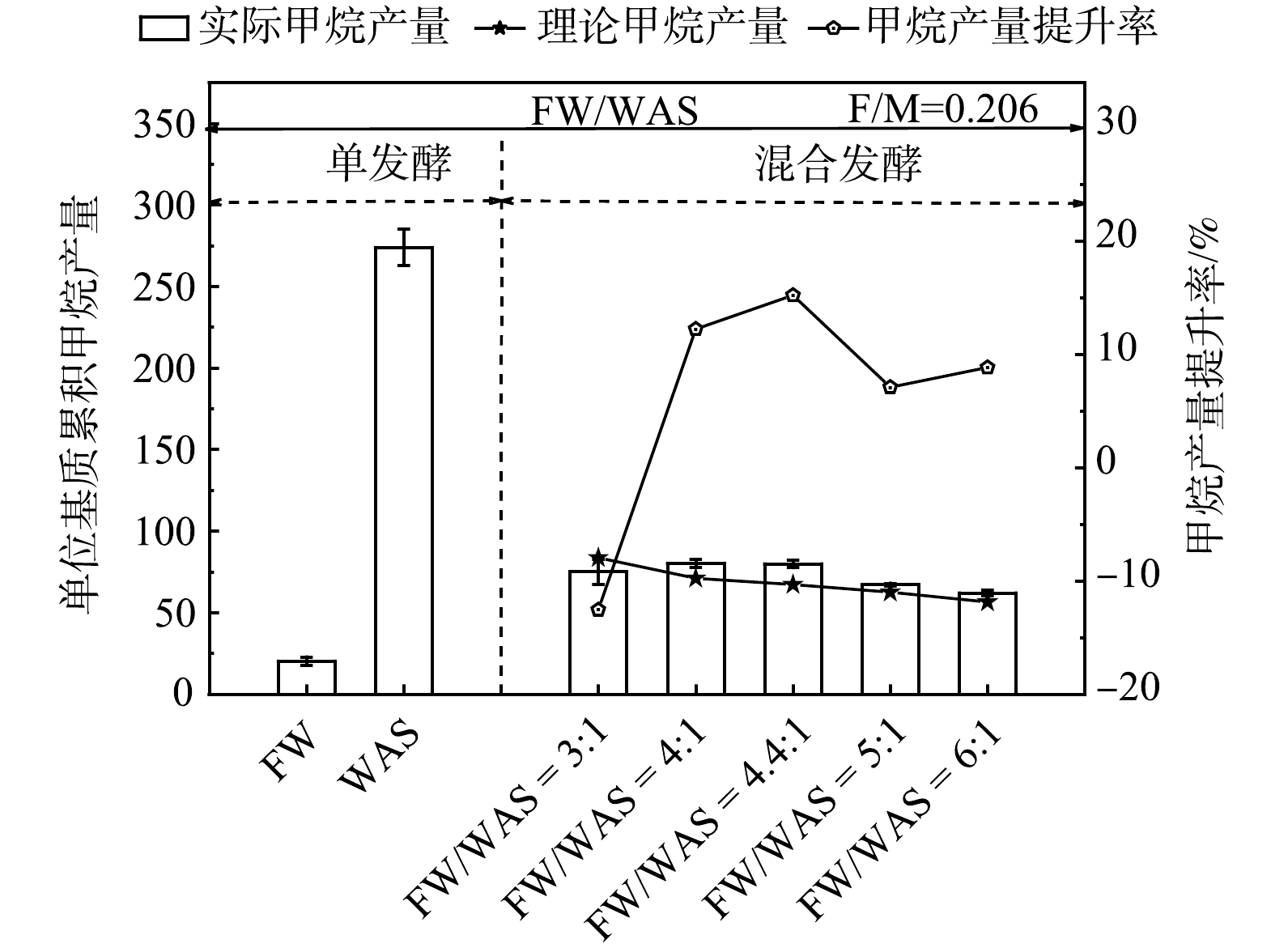
1) FW/WAS的优化。如表3所示,一级动力学模型和修正的Gompertz模型的拟合相关系数分别为0.971~0.991和0.975~0.987。这表明,2者均可较好地拟合FW和WAS厌氧发酵系统的累积产甲烷量。FW和WAS混合发酵的t0值趋近于0,表明FW和WAS混合发酵产甲烷基本无延滞期。在F/M为0.206条件下,不同FW/WAS的单位基质累积产甲烷量如图5所示。当厌氧发酵时间约为15 d时,FW/WAS等于4∶1和4.4∶1的单位基质累积产甲烷量明显高于3∶1、5∶1和6∶1时的单位基质累积产甲烷量。这表明,FW/WAS等于4∶1或4.4∶1时,FW和WAS混合发酵产甲烷的互促效果最佳。在FW/WAS为4∶1和4.4∶1时,运用Gompertz模型拟合分析可得P0和Rmax,如表3所示。可看出,在4.4∶1时,可获得更高的产甲烷潜能和最大生物气产率。如图6所示,当FW/WAS为4∶1和6∶1外,混合发酵的实际甲烷产率相对于单独发酵的加权平均值 (即理论甲烷产量) 均有不同程度的提升 (7.1%~15.2%)。其中,FW/WAS为4.4∶1时,相应的甲烷产量提升率最高。对比先前优化结果可发现[1],FW和WAS厌氧混合发酵系统经过长期驯化,最优基质混合比由初始最优值4∶1逐渐变为4.4∶1。因此,定期调整优化FW/WAS有利于厌氧混合发酵系统获得更高的产甲烷效能。
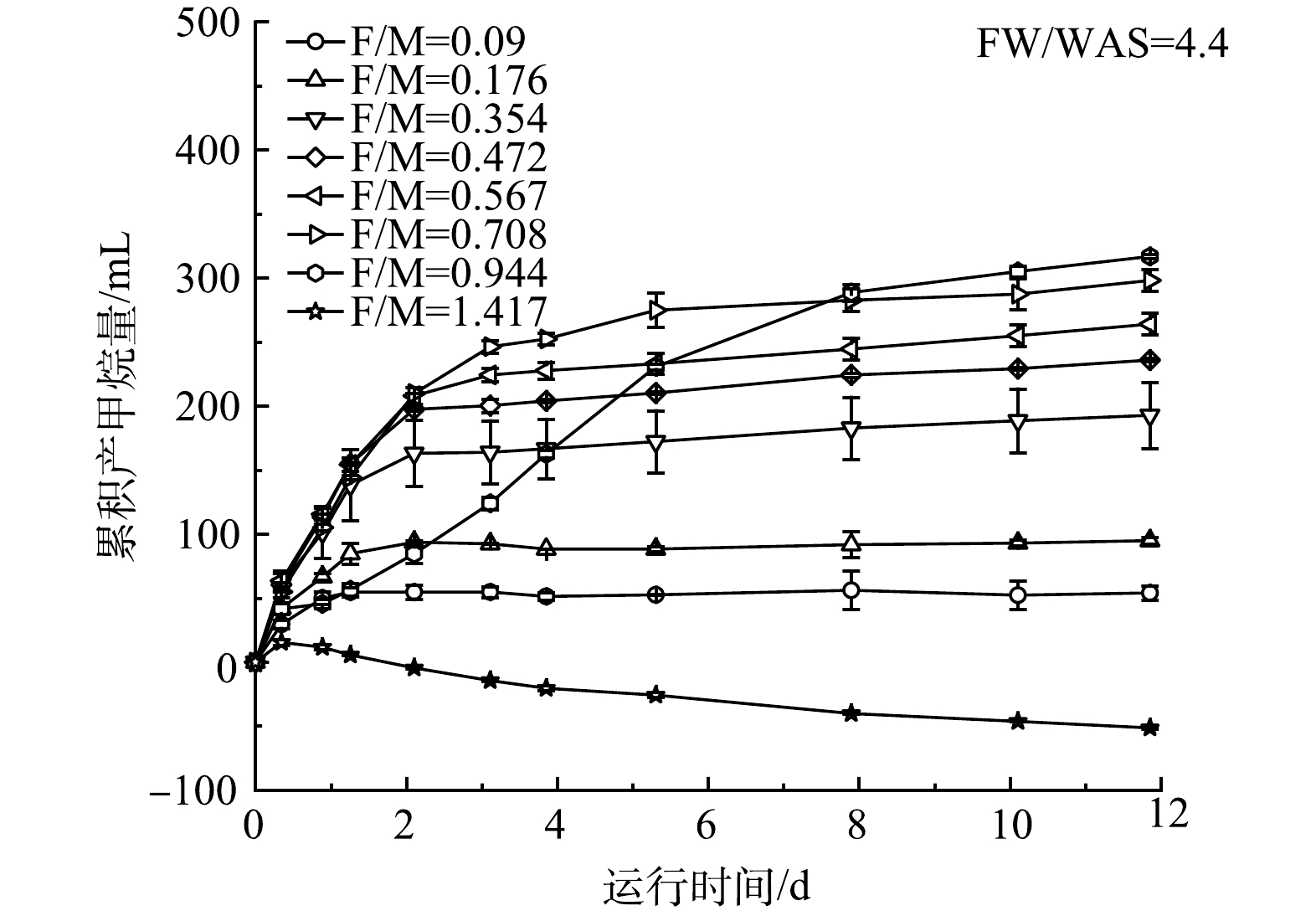
表 3 不同FW/WAS和F/M通过修正Gompertz模型和一级动力学模型拟合后产甲烷性能参数Table 3. Kinetic parameters of CH4 production with respect to different FW/WAS and F/M obtained from the modified Gompertz model and first-order model实验项目 FW/WAS F/M 修正的Gompertz模型 一级动力学模型 P0/mL Rmax/mL t0/d R2 P0/mL k/d-1 R2 FW单发酵 1∶0 0.206 16 4 0.2 0.975 17 0.287 0.971 WAS单发酵 0∶1 0.206 325 22 0.7 0.984 344 0.022 0.988 FW/WAS混合发酵 3∶1 0.206 70 6 0 0.984 74 0.160 0.993 FW/WAS混合发酵 4∶1 0.206 78 10 0 0.982 86 0.169 0.989 FW/WAS混合发酵 4.4∶1 0.206 82 11 0 0.985 88 0.172 0.994 FW/WAS混合发酵 5∶1 0.206 67 9 0 0.987 74 0.179 0.990 FW/WAS混合发酵 6∶1 0.206 63 8 0 0.985 68 0.181 0.991 F/M混合发酵 4.4∶1 0.090 51 105 0 0.985 51 2.610 0.977 F/M混合发酵 4.4∶1 0.176 91 85 0 0.979 91 1.610 0.989 F/M混合发酵 4.4∶1 0.354 166 99 0 0.969 169 0.968 0.981 F/M混合发酵 4.4∶1 0.472 219 126 0 0.980 223 0.874 0.987 F/M混合发酵 4.4∶1 0.567 240 118 0 0.982 246 0.751 0.990 F/M混合发酵 4.4∶1 0.708 277 106 0 0.989 286 0.575 0.996 F/M混合发酵 4.4∶1 0.944 325 43 0.02 0.994 402 0.135 0.984 F/M混合发酵 4.4∶1 1.417 0 0 2.0 0.902 0 0 0 2) F/M实验。将FW/WAS的最优值4.4∶1作为基质混合比,使用相同接种物评价F/M的影响。不同F/M下,FW和WAS厌氧发酵系统的累积产甲烷量如图7所示。当厌氧发酵时间约为12 d,F/M分别为0.09、0.176、0.354、0.472、0.567、0.708和0.944时,相应的甲烷产量对应为54.0、94.8、192、236、264、298和317 mL。如表3所示,运用Gompertz模型模拟分析可知相应的产甲烷潜能分别为51、91、166、219、240、277和325 mL,模型拟合相关系数为0.969~0.994,这表明拟合结果与实际吻合较好。此外,FW和WAS混合发酵的t0值也都趋于0,与前述结果一致。如图7和表3所示,当F/M为1.42时,累积产甲烷量和Rmax均为负值,这表明该结果无法用一级动力学模型和Gompertz模型拟合。其原因在于,在此负荷下,产甲烷菌的活性受到严重抑制。当F/M由0.090增至0.944时,累积产甲烷量和P0逐渐增加。当F/M为0.944时,与F/M为0.708相比,Rmax由106 mL降至43 mL,k由0.575 d−1降为0.135 d−1,分别降低了59.8%和76.5%。这表明,当F/M>0.708时,FW和WAS 混合发酵产甲烷的速率减缓。综上,FW和WAS厌氧混合发酵的最大耐受F/M为0.944,且当F/M>0.708时,相应的产甲烷速率减缓。
3. 结论
1) 在较低的有机负荷条件下能够实现连续流FW和WAS厌氧动态膜混合发酵系统的启动及其长期稳定运行,且系统碱度缓冲能力强、无酸累积,系统甲烷产量稳定。
2) 在连续流厌氧动态膜系统启动和长期运行过程中,能短时间形成动态膜,且对TCOD、蛋白质和多糖具有良好的截留率 (>95%) ,固液分离效果显著且能实现低浊度出料 (<50 NTU) 。
3) 厌氧动态膜混合发酵系统长期运行后,最优混合基质比为4.4∶1,同时,该系统的最大食微比为0.944,为该系统后续运行效能的优化提升提供了调控依据,以最大限度的快速实现连续流动态膜混合发酵系统的高效稳定运行。
-
表 1 鸡粪与接种污泥的性质
Table 1. Properties of chicken manure and inoculated sludge
% 实验材料 TS VS pH C N 粗蛋白 粗脂肪 粗纤维 钙 鸡粪 27.29 23.33 − 46.07 4.73 22.34 2.44 10.72 10.00 接种污泥 18.12 8.36 6.41 − − − − − − 表 2 中高温厌氧消化水解酸化细菌α多样性变化
Table 2. Changes in the α diversity of acidified bacteria during mid-high temperature anaerobic digestion
组数 物种数/个 均匀度指数 多样性指数 生物覆盖率 Shannon Simpson Chao1 ACE M1 1593 6.432 0.934 1816.911 1898.458 0.993 M2 1742 7.819 0.987 1946.074 2056.014 0.993 M3 1293 7.341 0.984 1460.754 1564.026 0.994 M4 1848 7.876 0.983 2075.346 2171.558 0.992 M5 1758 7.461 0.970 2013.739 2124.699 0.992 M6 1857 7.474 0.968 2108.730 2193.657 0.992 M7 1756 6.936 0.962 2059.034 2163.817 0.991 M8 1869 7.331 0.973 2125.733 2238.300 0.992 H1 2245 8.053 0.979 2538.160 2607.659 0.991 H2 2056 7.666 0.980 2365.965 2487.267 0.990 H3 1921 7.275 0.976 2232.204 02382.96 0.990 H4 2391 8.597 0.991 2650.590 2772.402 0.990 H5 1897 6.837 0.961 2194.613 2335.348 0.990 H6 1615 7.161 0.978 1880.154 2005.092 0.992 H7 1769 6.784 0.898 1964.061 2037.415 0.992 H8 1634 6.802 0.968 1905.350 2043.547 0.991 表 3 中高温厌氧消化产甲烷古菌α多样性变化
Table 3. Variation of alpha diversity of methanogenic archaea during mid-high temperature anaerobic digestion
组数 物种数/个 均匀度指数 多样性指数 生物覆盖率 Shannon Simpson Chao1 ACE M1 533 4.109 0.822 602.843 620.164 0.998 M2 584 5.413 0.946 631.616 644.214 0.999 M3 544 5.315 0.947 602.235 611.049 0.999 M4 514 5.456 0.951 569.836 573.337 0.999 M5 624 5.742 0.962 669.764 685.770 0.999 M6 316 4.421 0.854 341.161 339.959 0.999 M7 191 3.617 0.812 217.400 218.061 1.000 M8 189 3.780 0.847 204.750 209.107 1.000 H1 446 5.027 0.932 497.679 528.585 0.999 H2 553 5.184 0.939 613.061 633.953 0.998 H3 483 5.244 0.946 516.514 527.499 0.999 H4 664 5.414 0.943 758.897 757.104 0.998 H5 530 5.322 0.941 587.554 588.961 0.999 H6 276 2.899 0.681 298.000 308.641 0.999 H7 162 2.984 0.786 193.231 183.107 1.000 H8 191 3.153 0.805 204.034 211.344 1.000 -
[1] 袁惊柱, 朱彤. 生物质能利用技术与政策研究综述[J]. 中国能源, 2018, 40(6): 16 − 20. [2] ZHANG LB, YANG T. The Evaluation and Selection of Renewable Energy Technologies in China[J]. Energy Procedia, 2014, 61: 2554 − 2557. doi: 10.1016/j.egypro.2014.12.044 [3] YAO Y, HUANG G, AN C, et al. Anaerobic digestion of livestock manure in cold regions: Technological advancements and global impacts[J]. Renewable and Sustainable Energy Reviews, 2020, 119: 109494. doi: 10.1016/j.rser.2019.109494 [4] LIU WR, ZENG D, SHE L, et al. Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China[J]. Science of The Total Environment, 2020, 734: 139023. doi: 10.1016/j.scitotenv.2020.139023 [5] 唐涛涛, 李江, 杨爱江, 等. 秸秆类型及配比变化对污泥厌氧消化中微生物群落的影响[J]. 化工进展, 2020, 39(2): 667 − 678. doi: 10.16085/j.issn.1000-6613.2019-0777 [6] 李霞. 当前我国畜牧养殖对生态环境的影响[J]. 农业与技术, 2016, 36(14): 239. [7] PONSA S, FERRER I, VAZQUEZ F, et al. Optimization of the hydrolytic-acidogenic anaerobic digestion stage (55°C) of sewage sludge: Influence of pH and solid content[J]. Water Research, 2008, 42(14): 3972 − 3980. doi: 10.1016/j.watres.2008.07.002 [8] 张文哲, 陈静, 刘玉, 等. 中温和高温厌氧消化的比较[J]. 化工进展, 2018, 37(12): 4853 − 4861. doi: 10.16085/j.issn.1000-6613.2018-0599 [9] 曲艺源, 张景新, 何义亮. 铁电极辅助餐厨垃圾高温厌氧消化及微生物的耐盐机理[J]. 化工进展, 2022, 41(4): 8. doi: 10.16085/j.issn.1000-6613.2021-0819 [10] MICOLUCCI F, GOTTARDO M , PAVAN P , et al. Pilot scale comparison of single and double-stage thermophilic anaerobic digestion of food waste[J]. Journal of Cleaner Production, 2017: 1376-1385. [11] KJERSTADIUS. H, JANSEN JL, DE V, et al. Hygienization of sludge through anaerobic digestion at 35, 55 and 60 degrees C[J]. Water Science and Technology, 2013, 68(10): 2234 − 2239. doi: 10.2166/wst.2013.486 [12] FERNANDEZRODRIGUEZ J, PEREZ M, ROMERO LI, et al. Comparison of mesophilic and thermophilic dry anaerobic digestion of OFMSW: Kinetic analysis[J]. Chemical Engineering Journal, 2013(232): 59 − 64. [13] WANG Z, MA T, XING L. Process performance and microbial interaction in two-stage continuously stirred tank reactors for sludge anaerobic digestion operated at different temperatures[J]. Biochemical Engineering Journal, 2020, 161: 107682. doi: 10.1016/j.bej.2020.107682 [14] 李金璐, 王硕, 于婧, 等. 2013. 一种改良的植物 DNA提取方法[J]. 植物学报, 2013, 48(1): 72 − 78. [15] KIM M S, KIM D H, YUN Y M. Effect of operation temperature on anaerobic digestion of food waste: performance and microbial analysis[J]. Fuel, 2017, 209: 598 − 605. doi: 10.1016/j.fuel.2017.08.033 [16] NGES I A, JING L. Effects of solid retention time on anaerobic digestion of dewatered-sewage sludge in mesophilic and thermophilic conditions[J]. Renewable Energy, 2010, 35(10): 2200 − 2206. doi: 10.1016/j.renene.2010.02.022 [17] YANG Z Y, WANG W, ZHANG SY, et al. Comparison of the methane production potential and biodegradability of kitchen waste from different sources under mesophilic and thermophilic conditions[J]. Water Science And Technology. 2017, 75(7), 1607-1616. [18] FOUNTOULAKIS M S, DRAKOPOULOU S, TERZAKIS S, et al. Potential for methane production from typical Mediterranean agro-industrial by-products[J]. Biomass & Bioenergy, 2008, 32(2): 155 − 161. [19] ALMEIDA S D. Comparison of the anaerobic digestion at the mesophilic and thermophilic temperature regime of organic wastes from the agribusiness[J]. Bioresource Technology, 2017, 241: 985. doi: 10.1016/j.biortech.2017.06.006 [20] ZHAO Y, SUN F, YU J, et al. Co-digestion of oat straw and cow manure during anaerobic digestion: Stimulative and inhibitory effects on fermentation[J]. Bioresource Technology, 2018, 269: 143 − 152. doi: 10.1016/j.biortech.2018.08.040 [21] DUAN N, ZHANG D J, LIN C, et al. Effect of organic loading rate on anaerobic digestion of pig manure: Methane production, mass flow, reactor scale and heating scenarios[J]. Journal of Environmental Management, 2019, 231: 646 − 652. doi: 10.1016/j.jenvman.2018.10.062 [22] HIDAKA T, WANG F, TOGARI T, et al. Comparative performance of mesophilic and thermophilic anaerobic digestion for high-solid sewage sludge[J]. Bioresource Technology, 2013, 149(12): 177 − 183. [23] GARCIA M L, ANGENENT L T. Interaction between temperature and ammonia in mesophilic digesters for animal waste treatment[J]. Water Research, 2009, 43(9): 2373 − 2382. doi: 10.1016/j.watres.2009.02.036 [24] 郭香麟, 左剑恶, 史绪川, 等. 餐厨垃圾与秸秆混合中温和高温厌氧消化对比[J]. 环境科学, 2017, 38(7): 3070 − 3077. doi: 10.13227/j.hjkx.201612267 [25] 宋壮壮, 吕爽, 刘哲, 等. 厌氧氨氧化耦合反硝化工艺的启动及微生物群落变化特征[J]. 环境科学, 2019, 40(11): 5057 − 5065. doi: 10.13227/j.hjkx.201905223 [26] BAKER A B, TAWABINI B, NAZAL M, et al. Efficiency of Thermophilic Bacteria in Wastewater Treatment[J]. Arabian Journal for Science and Engineering, 2021, 46(1): 123 − 128. doi: 10.1007/s13369-020-04830-x [27] HE C. , ZHANG BG, YAN WY, et al. Enhanced Microbial Chromate Reduction Using Hydrogen and Methane as Joint Electron Donors[J]. Journal of Hazardous Materials, 2020(395): 122648. [28] PENG X, ZHANG S, LI L, et al. Long-term high-solids anaerobic digestion of food waste: Effects of ammonia on process performance and microbial community[J]. Bioresource Technology, 2018, 262: 148 − 158. doi: 10.1016/j.biortech.2018.04.076 [29] 李旭, 冯磊, 甄箫斐, 等. 基于CSTR反应器鸡粪秸秆共消化产甲烷特性及菌群变化研究[J]. 环境科学学报, 2021, 41(08): 3312 − 3323. doi: 10.13671/j.hjkxxb.2021.0036 [30] LIN L, YU Z, LI Y. Sequential batch thermophilic solid-state anaerobic digestion of lignocellulosic biomass via recirculating digestate as inoculum – Part II: Microbial diversity and succession[J]. Bioresource Technology, 2017, 241: 1027 − 1035. doi: 10.1016/j.biortech.2017.06.011 [31] SUN LW, TOYONAGA M, OHASHI A, et al. Lentimicrobium saccharophilum gen. nov., sp nov., a strictly anaerobic bacterium representing a new family in the phylum Bacteroidetes, and proposal of Lentimicrobiaceae fam. nov.[J]. International Journal of Systematic and Evolutionary Microbiology, 2016, 66(7): 2635 − 2642. doi: 10.1099/ijsem.0.001103 [32] HANIA W B, GODBANE R, POSTEC A, et al. Defluviitoga tunisiensis gen. nov. sp. nov. a thermophilic bacterium isolated from a mesothermic and anaerobic whey digester[J]. International Journal of Systematic and Evolutionary Microbiology, 2012, 62: 1377 − 1382. doi: 10.1099/ijs.0.033720-0 [33] MAUS I. , KOECK D. E., CIBIS K. G., et al. Unraveling the microbiome of a thermophilic biogas plant by metagenome and metatranscriptome analysis complemented by characterization of bacterial and archaeal isolates[J]. Biotechnology for Biofuels, 2016, 9: 171. doi: 10.1186/s13068-016-0581-3 [34] WU ZY. , NGUYEN D., LAM TY., et al. Synergistic association between cytochrome bd-enSCODed Proteiniphilum and reactive oxygen species (ROS)-scavenging methanogens in microaerobic-anaerobic digestion of lignocellulosic biomass[J]. Water Research, 2021, 190: 116721. doi: 10.1016/j.watres.2020.116721 [35] KOECK D E., HAHNKE S., ZVERLOV VV. Herbinix luporum sp nov., a thermophilic cellulose-degrading bacterium isolated from a thermophilic biogas reactor[J]. International Journal of Systematic and Evolutionary Microbiology, 2016, 66(10): 4132 − 4137. doi: 10.1099/ijsem.0.001324 [36] ESQUIVEL-ELIZONDO S. , BAGIC C., TEMOVSKA M., et al. The Isolate Caproiciproducens sp. 7D4C2 Produces n-Caproate at Mildly Acidic Conditions From Hexoses: Genome and rBOX Comparison With Related Strains and Chain-Elongating Bacteria[J]. Frontiers in Microbiology, 2021, 11: 594524. doi: 10.3389/fmicb.2020.594524 [37] TIAN GL, YANG B, DONG MH, et al. The effect of temperature on the microbial communities of peak biogas production in batch biogas reactors[J]. Renewable Energy, 2018, 123: 15 − 25. doi: 10.1016/j.renene.2018.01.119 [38] KRAUSE L. , DIAZ N N, EDWARDS R A, et al. Taxonomic composition and gene content of a methane-producing microbial community isolated from a biogas reactor[J]. Journal and Biotechnology, 2008, 136(1-2): 91 − 101. doi: 10.1016/j.jbiotec.2008.06.003 [39] 杨冰, 卢向阳, 田云. 甲烷八叠球菌研究进展[J]. 化学与生物工程, 2012, 29(12): 7 − 11. doi: 10.3969/j.issn.1672-5425.2012.12.002 [40] MBADINGA SM. , LI KP., ZHOU L. Analysis of alkane-dependent methanogenic community derived from production water of a high-temperature petroleum reservoir[J]. Applied Microbiology and Biotechnology, 2012, 96(2): 531 − 542. doi: 10.1007/s00253-011-3828-8 [41] 麻婷婷, 承磊, 刘来雁, 等. 不同抑制剂对乙酸降解产甲烷及产甲烷菌群结构的影响[J]. 微生物学报, 2015, 55(5): 587 − 597. doi: 10.13343/j.cnki.wsxb.20140499 [42] 盛多红. 超嗜热古菌基因组的热稳定性[J]. 生命科学, 2014, 26(1): 64 − 71. doi: 10.13376/j.cbls/2014010 [43] SUTER B, GRAHAM C, STAGLJAR I. Exploring protein phosphorylation in response to DNA damage using differentially tagged yeast arrays[J]. Biotechniques, 2008, 45(5): 581 − 584. doi: 10.2144/000112949 [44] DAI Y, GRANT S. New insights into checkpoint kinase 1 (Chk1) in the DNA damage response (DDR) signaling network: Rationale for employing Chk1 inhibitors in cancer therapeutics[J]. Clin Cancer Res, 2010, 16(2): 376 − 383. doi: 10.1158/1078-0432.CCR-09-1029 [45] KUNDU K, SHARMA S, SREEKRISHNAN T R. Changes in microbial communities in a hybrid anaerobic reactor with organic loading rate and temperature[J]. Bioresource Technology, 2013, 129(2): 538 − 547. [46] GAO W J, LEUNG K T, QIN W S, et al. Effects of temperature and temperature shock on the performance and microbial community structure of a submerged anaerobic membrane bioreactor[J]. Bioresource Technology, 2011, 102(19): 8733 − 8740. doi: 10.1016/j.biortech.2011.07.095 [47] LIN Q, HE GH, RUI JP. , et al. Microorganism-regulated mechanisms of temperature effects on the performance of anaerobic digestion[J]. Microbal Cell Factories, 2016, 15: 96. doi: 10.1186/s12934-016-0491-x [48] WITTEBOLLE L, MARZORA TI M, CLEMENT L, et al. Initial community evenness favours functionality under selective stress[J]. Nature, 2009, 458(7238): 623. doi: 10.1038/nature07840 [49] 潘婧冉, 高苏, 赵国柱, 等. 餐厨垃圾厌氧消化处理主要过程的微生物群落结构分析[J]. 微生物学通报, 2019, 46(11): 2886 − 2899. doi: 10.13344/j.microbiol.china.181016 [50] ROS M. , OLIVEIRA JD., MURCIA MDP, et al. Mesophilic anaerobic digestion of pig slurry and fruit and vegetable waste: Dissection of the microbial community structure[J]. Journal of Cleaner Production, 2017, 156: 757 − 765. doi: 10.1016/j.jclepro.2017.04.110 [51] GUO X, CHENG W, SUN F, et al. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings[J]. Bioresource Technology, 2014, 152: 420. doi: 10.1016/j.biortech.2013.11.012 -




 下载:
下载: