-
随着工业、经济发展和城市化进程的加快与人民生活水平的逐渐提高,我国水资源供需矛盾日益突出。我国水资源年平均总量大约为2.8亿m3,人均水资源量为2 200 m3,仅相当于世界人均水资源量的1/4,被列为世界上人均水资源量最匮乏的13个国家之一[1]。针对我国水资源短缺,污水资源丰富但污水再生利用量不足的现状,实行再生水循环利用是最有效的解决方法[2]。再生水不同利用途径对再生水质的要求不同[3],景观环境用水在城市用水中占较大比例[4],因此合理选择再生水处理技术使再生水回用于景观用水很有必要。
目前,再生水处理技术主要有混凝沉淀[5-6]、吸附[7-8]、臭氧氧化[9-10]、人工湿地[11-12]等。韩玉珠[13]等用混凝沉淀法处理二沉池出水时,发现在消石灰投加量为500 mg/L且三氯化铁投加量为15 mg/L,出水水质满足景观用水水质标准。李夏青[14]等用臭氧氧化法处理二级生化处理出水时,发现臭氧投加量为4.1 mg/L且反应时间为10 min时,出水水质满足景观环境用水水质标准。混凝沉淀法在混凝过程中易被混凝剂干扰,处理效果不稳定。臭氧氧化法与吸附法相比,经济成本较高。污水厂尾水B/C较低,不宜采用生化法。因此本实验选择吸附法处理污水厂尾水,吸附剂为活性炭,活性炭具有高比表面积[15]吸附性能好[16]且价格低廉等特点。
本研究首先采用单因素实验法,考察不同影响因素对活性炭吸附处理污水厂尾水效果,然后进一步研究活性炭吸附的动力学,最后通过采用三维荧光光谱法及荧光区域积分分析 (FRI)对二沉池出水进行定量分析,考察活性炭吸附效果,为活性炭吸附应用于尾水回用景观用水提供理论依据。
-
实验用活性炭为市售木质粉末活性炭,比表面积为1 000 m2/g,碘值为500~1 200 mg/L,亚甲基蓝吸附为180 mg/g。
实验用水取自曲阜某污水厂二沉池出水,污水厂出水执行《城镇污水处理厂污染物排放标准:GB18918—2022》中的一级A标准,实验用水具体水质参数,见表1。实验用水用0.45 μm醋酸纤维膜过滤后,放入4 ℃的恒温冰箱中保存,确保实验水质稳定。
-
采用美国-Therno fisherNilet iS50傅立叶变换红外光谱仪表征木质活性炭内部官能团。首先,称取适量活性炭样品放入玛瑙研钵中,研磨后放入105 ℃烘箱干燥。然后称取适量色谱纯KBr放入玛瑙研钵中,研磨后压片作为空白样,用红外光谱测定空白样的背景值。最后,取干燥后的活性炭粉末适量,加入适量色谱纯KBr研磨均匀后压片,检测时波数范围选择在400~4 000 cm−1之间,检测样品的红外光谱特征。
-
取200 mL水样于5个烧杯中,置于六联数显电动搅拌器,研究活性炭吸附过程的最佳实验条件。分别调节活性炭投加量、吸附时间、初始pH和温度,以快速混合搅拌作为反应起点,反应完毕后静置沉淀30 min,使用虹吸法吸取上清液 (液面下1~2 cm处),测定COD、NH4+-N、TN和TP浓度。
-
实验前,将木质活性炭洗净烘干(105 ℃,6 h) 后,置于真空干燥器皿中备用。实验水样用0. 45 μm醋酸纤维膜过滤后,存于4 ℃冰箱。
-
水质指标测定方法参照《水和废水监测分析方法(第4版)》。pH采用pH计测定,总氮采用碱式过硫酸钾消解紫外分光光度法测定,总磷采用钼酸铵分光光度法测定,氨氮采用纳氏试剂分光光度法测定,化学需氧量使用哈希DR-1900测定。三维荧光光谱采用日立(Hitachi)F-7000荧光分光光度计测定。
-
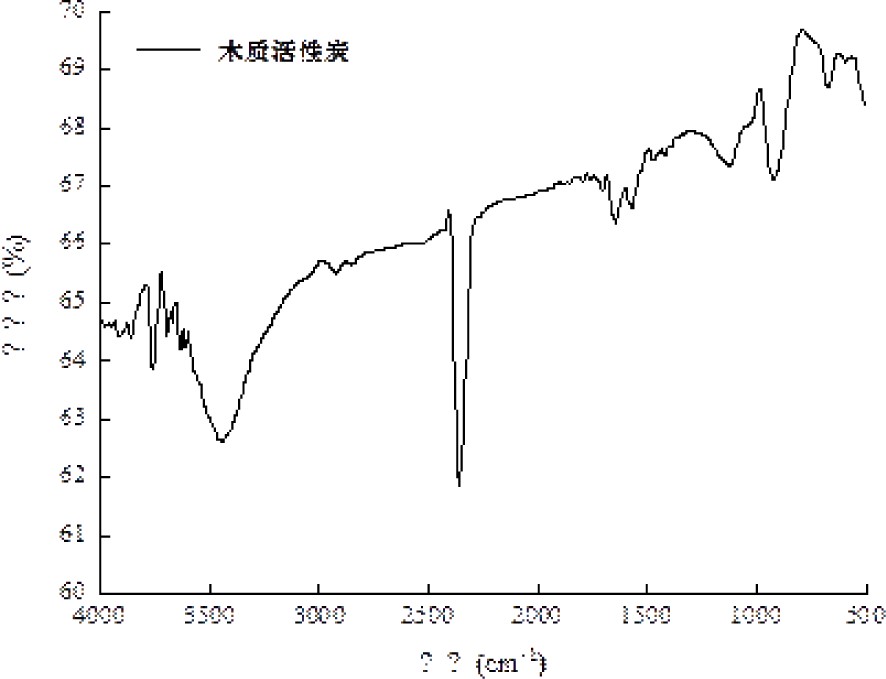
木质活性炭的红外吸收光谱,见图1。
图1可知,可以发现有多个吸收峰,说明在活性炭中有丰富的官能团结构。在3 451 cm−1处有明显的吸收振动峰,主要是由于醇酚羟基或者化学吸附水性O—H的伸缩振动引起的。在2 350 cm−1有明显的吸收振动峰,主要由双键(=C=C=C=)和三键(—C≡C—)引起的[17]。由此可知,因为这些基团的存在,使得活性炭具有良好的吸附能力。
-
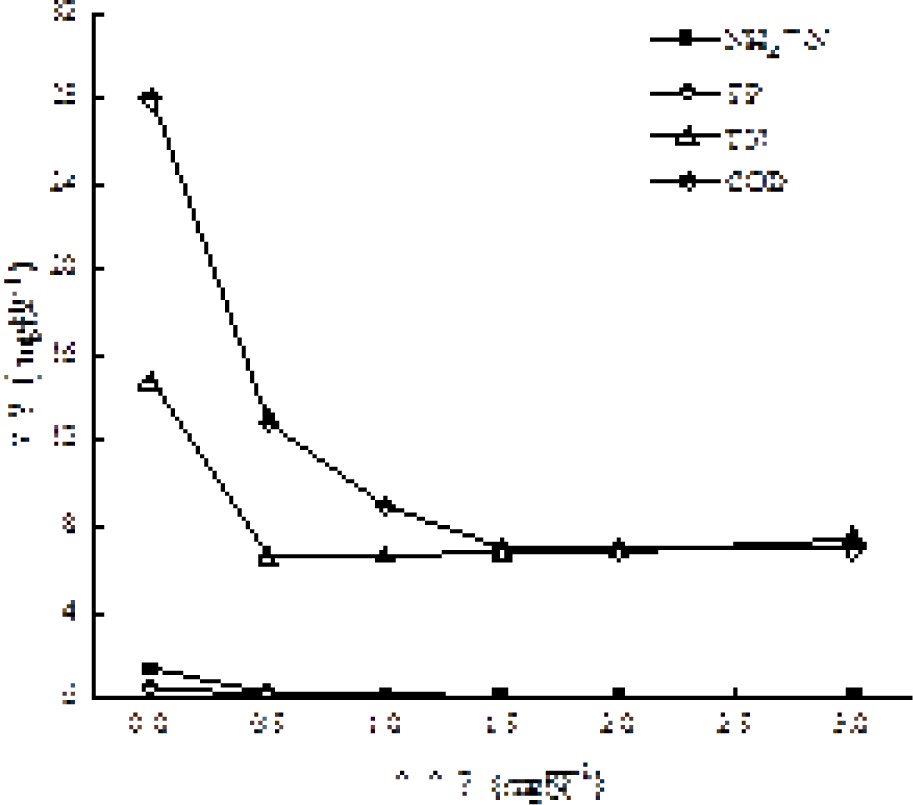
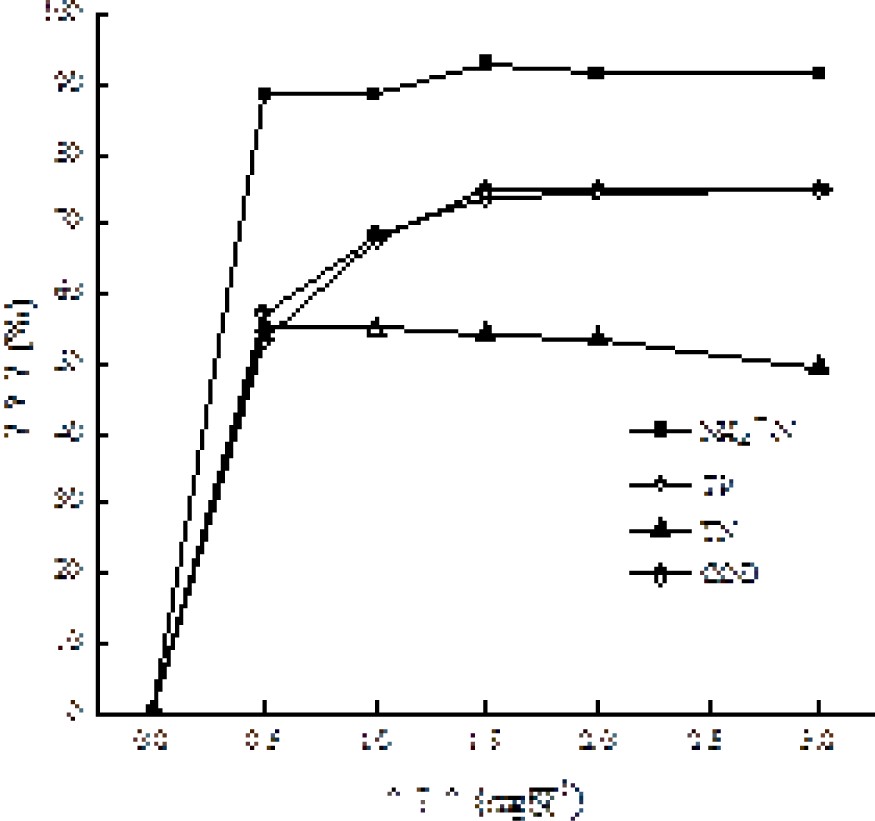
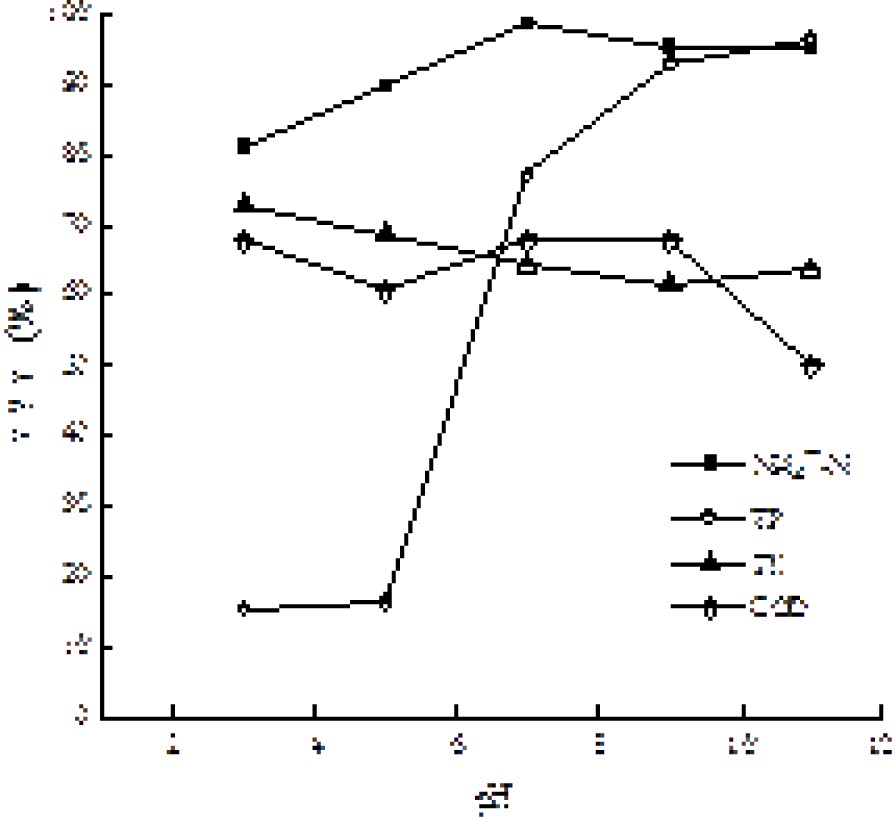
在实验用水体积为200 mL,初始pH为7,反应温度为25 ℃,反应时间为60 min,活性炭投加量分别在0.5、1.0、1.5、2.0和3.0 g/L的条件下,通过对COD、NH4+-N、TN和TP去除效果,考察活性炭投加量对吸附效果和去除效果的影响,见图2和图3。
图2和图3可知,随着活性炭投加量的不断增大,出水NH4+-N、TP、TN和COD浓度呈现出逐渐降低的趋势,当活性炭的投加量为1.5 g/L时,NH4+-N、TP、TN和COD去除率最大且满足《城市污水再生利用 景观环境用水水质:GB/T 18921—2019》标准,再继续增大活性炭投加量,浓度降低幅度逐渐变小,说明吸附达到平衡状态,再增加活性炭用量已没有意义。唐安琪[18]在活性炭处理煤化工废水静态吸附试验中表明活性炭投加量越多,去除效果越明显,但继续增加活性炭投加量,去除效果不明显,与本实验结果相符。从吸附效果和活性炭经济成本综合考虑,本实验控制活性炭的最佳投加量为1.5 g/L。
理论上,随着活性炭用量的增加,提供的吸附点位数越多,吸附效果越好。本实验出现这种现象的原因是:随着活性炭投加量增加,吸附表面积增加,活性炭上可供吸附的活性点位数量也增大,因此吸附量增加[19]。但当活性炭投加过量时,随吸附表面积和吸附活性点位数的增加,活性炭吸附单位容量减小,因此随活性炭过量增加时,浓度降低速度减缓而趋于稳定。
-
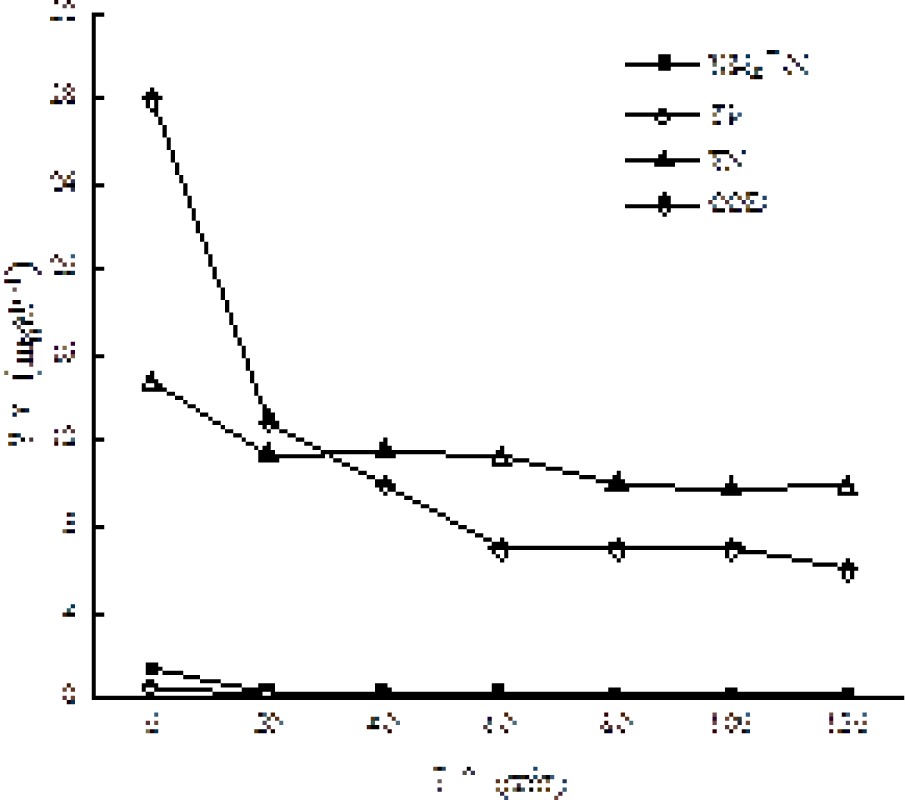
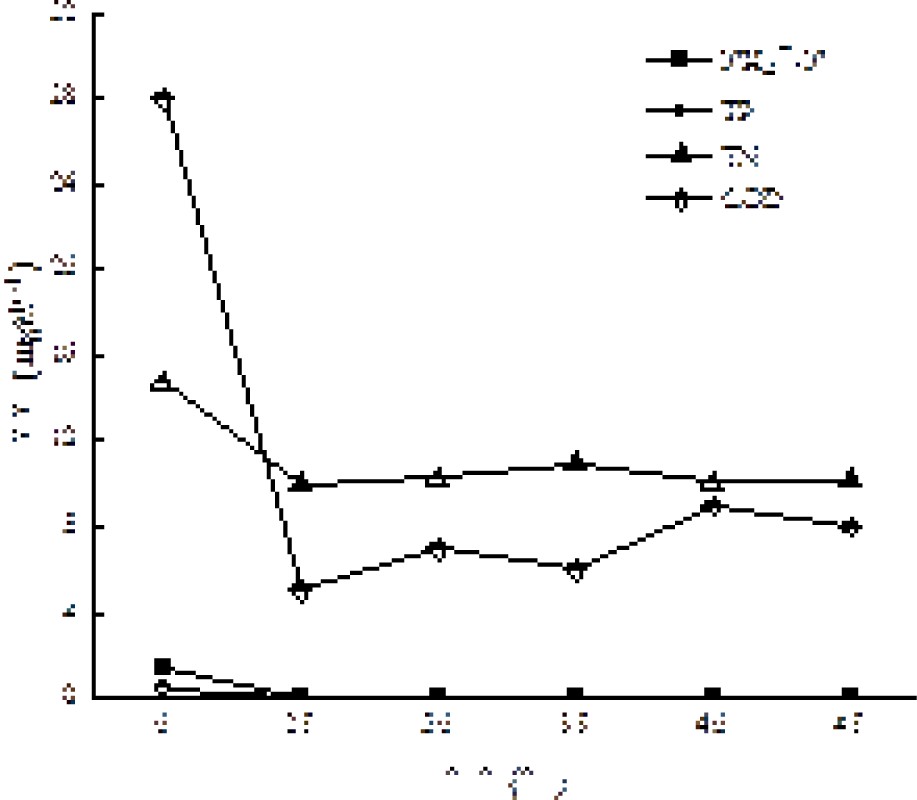
在原水体积为200 mL,初始pH为7,反应温度为25 ℃,投加量为1.5 g/L,吸附时间为20、40、60、80、100、120 min条件下,通过对COD、NH4+-N、TN和TP去除效果,考察活性炭吸附时间对吸附效果和去除效果的影响,见图4和图5。
图4和图5可知,随着吸附时间增加,出水NH4+-N、TP、TN和COD浓度逐渐降低。 经活性炭吸附80 min后,NH4+-N、TP、TN和COD满足《城市污水再生利用 景观环境用水水质:GB/T 18921—2019》标准。再继续延长吸附时间,浓度降低幅度逐渐变小,吸附达到平衡状态。因此本实验控制活性炭的吸附时间为80 min。
本实验出现这种现象的原因是:吸附在活性炭上污染物的量随着时间的延长而增加,导致活性炭表面的有效点位减少,当有效点位趋于零时,体系已达到吸附平衡[20]。
-
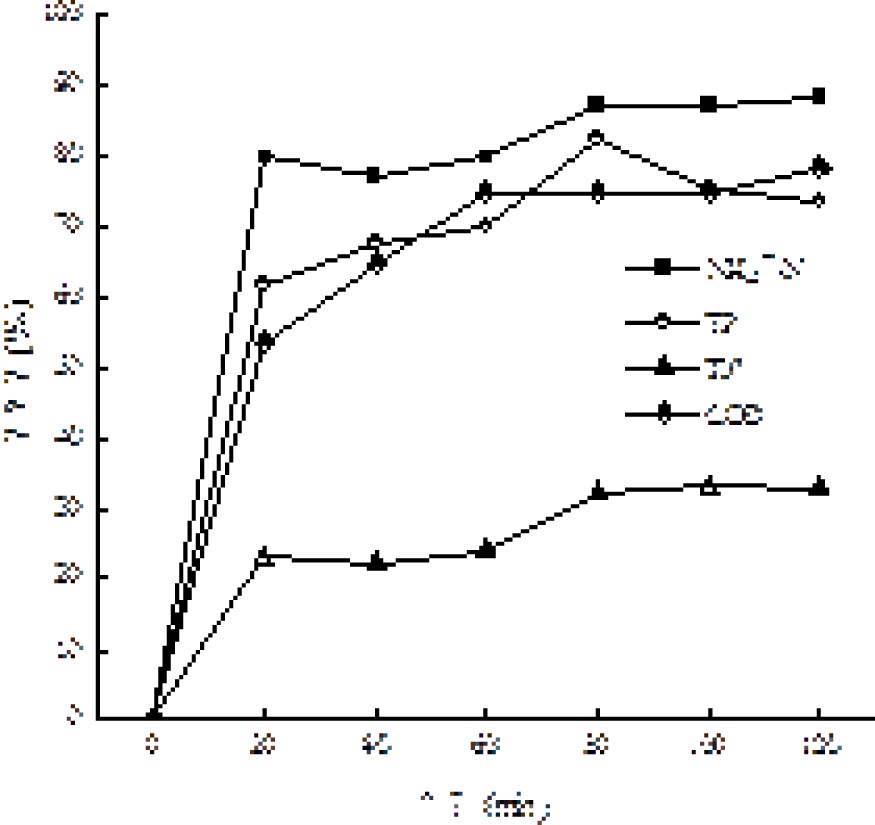
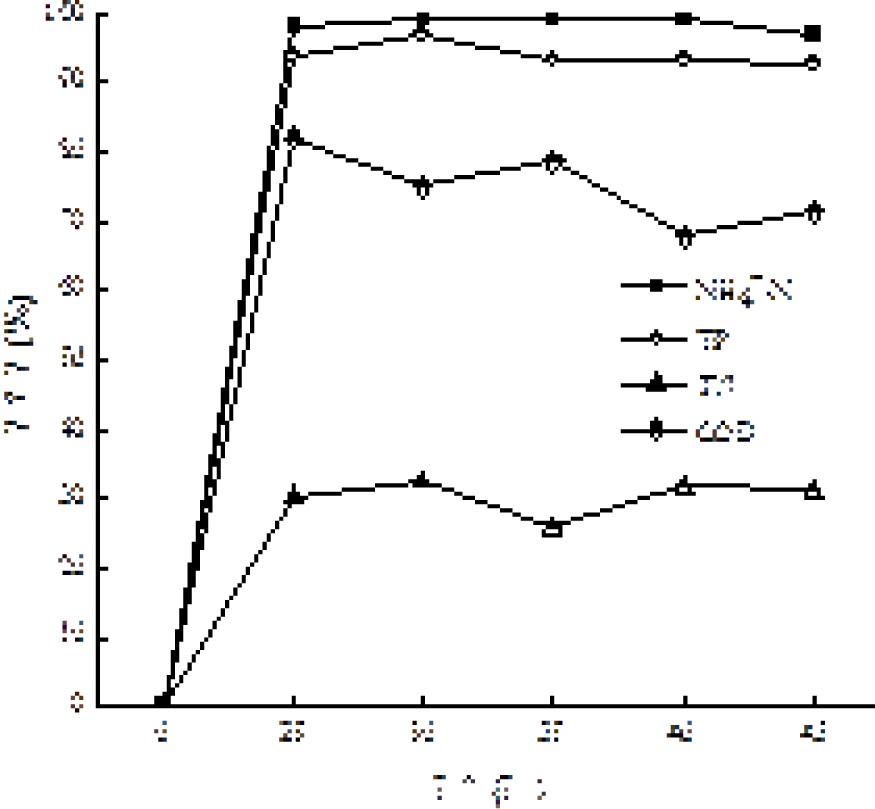
在原水体积为200 mL,反应温度为25 ℃,投加量为1.5 g/L,吸附时间为80 min,初始pH为3、5、7、9、11的条件下,通过对COD、NH4+-N、TN和TP去除效果,考察初始pH值对吸附效果和去除效果的影响,见图6和图7。
图6和图7可知,随着pH增加,出水NH4+-N和TP浓度逐渐降低,TN浓度逐渐增加但增加幅度不大,COD浓度先降低后增加。在pH为7时,NH4+-N、TP、TN和COD满足《城市污水再生利用 景观环境用水水质:GB/T 18921—2019》标准。因此本实验控制初始pH为7。殷玉蓉等[21]在使用活性炭对污水厂尾水深度处理时,发现pH在中性范围内有较高吸附率,这与实验结果相符。
本实验TN和COD浓度出现这种现象的原因是:当溶液pH偏酸性时,溶液中存在的大量H+离子,此时活性炭的有效吸附点位被H+离子占据,因为H+离子会与活性炭表面的官能基团结合,使活性炭没有足够的吸附位点来与污染物结合。当溶液pH慢慢偏碱性时,H+被越来越多的OH-所中和,这时与活性炭表面官能团结合的H+发生解离,使得之前被H+占据的活性点位被暴露出来,重新被污染物占据。但当溶液中pH过大时,溶液中存在大量的OH-离子,它会阻碍污染物离子的扩散。
-
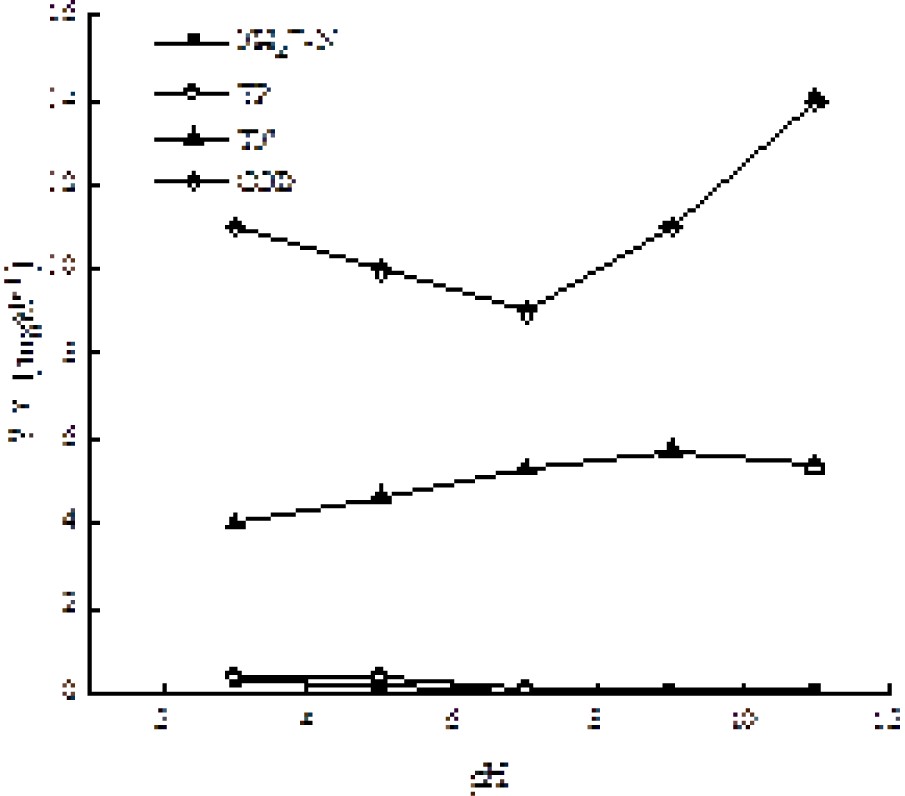
在原水体积为200 mL,投加量为1.5 g/L,吸附时间为80 min,pH为7,温度为25、30、35、40、45 ℃的条件下,通过对COD、NH4+-N、TN和TP去除效果,考察温度对吸附效果和去除效果的影响,见图8和图9。
图8和图9可知,随着温度增加,出水NH4+-N、TPP、TN和COD浓度逐渐降低但降低幅度不大,结果表明温度对木质活性炭吸附效果影响较小。在温度为25 ℃时,NH4+-N、TP、TN和COD满足《城市污水再生利用 景观环境用水水质:GB/T 18921—2019》标准,NH4+-N、TP、TN和COD去除率分别为98.01%、94.04%、32.40%和82.14%。因此本实验控制温度为25 ℃。
-
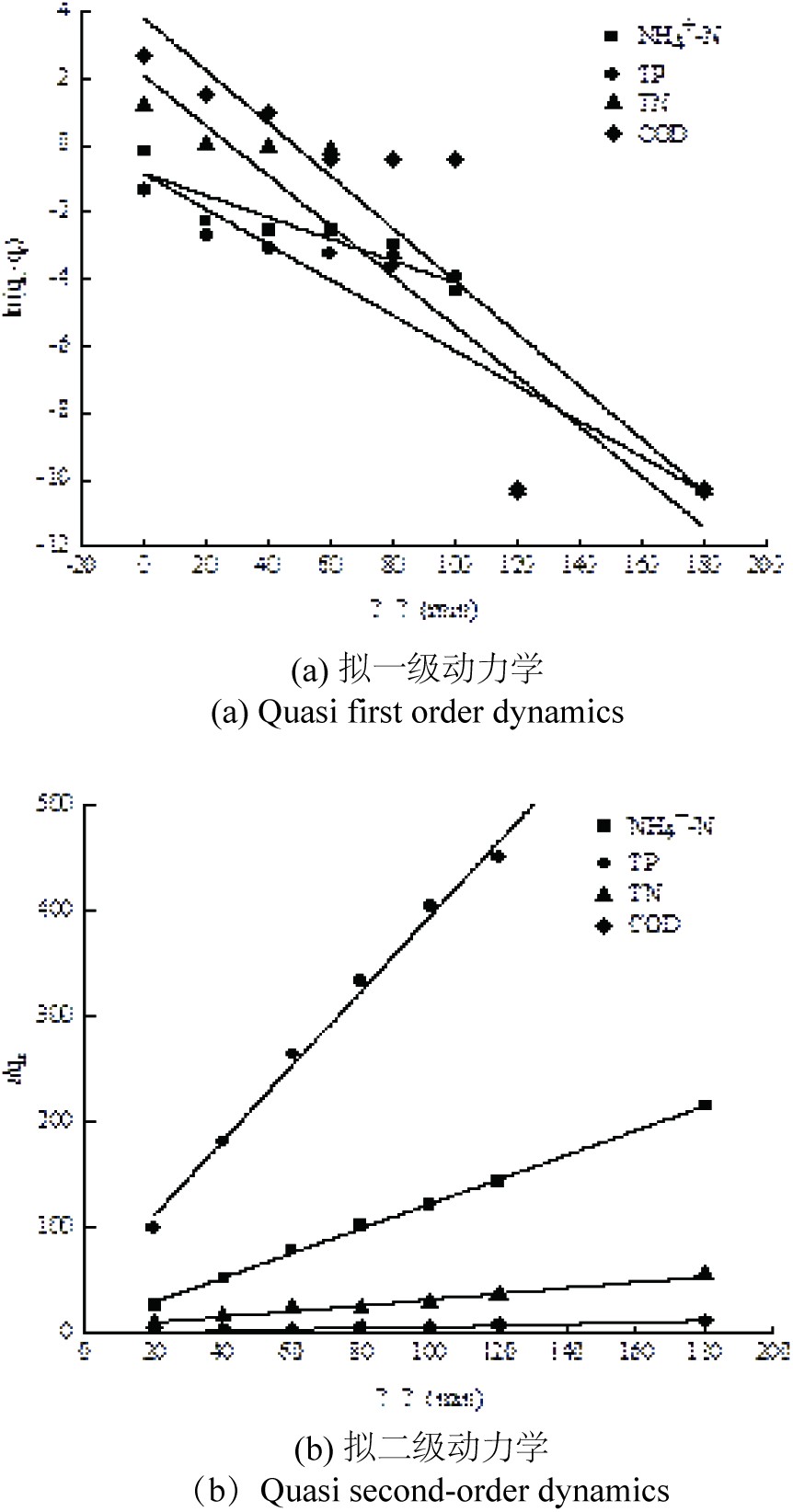
为了研究活性炭吸附NH4+-N、TP、TN和COD的动力学特性,拟采用Langergren拟一级动力学方程和拟二级动力学方程对吸附动力学进行拟合,通过相关系数来判断两种动力学方程能够更加准确地反映出动力学过程。
根据边界条件t=0、qt=0及 t=t、qt=qt积分,见式(2):
式中: t为吸附时间,min; qe和qt分别为吸附平衡时及t时刻的吸附剂对吸附质的吸附量,mg/g;k1为拟一级动力学模型的吸附速率常数,min−1。以ln( qe-qt ) 对t作图得到直线,通过直线的斜率和截距可以计算出参数k1和qe的值。
根据同样的边界条件,其积分表达,见式(4):
式中: k2为拟二级动力学模型的吸附速率常数,g·(mg·min)−1;其他与式(2)相同。以t /qt对t作图得到直线,通过直线的斜率和截距可以计算出参数qe和k2的值。
根据拟一级动力学模型方程(1)和拟二级动力学模型方程(2),将吸附量与时间的关系进行拟合。经过动力学模型对活性炭吸附水中NH4+-N、TP、TN和COD的拟合,拟一级动力学方程、拟二级动力学方程的拟合,见图10和表2。
由拟合结果可知,NH4+-N、TP、TN和COD的拟一级动力学方程和相关性系数都低,拟二级动力学拟合相关系数分别为0.998 7、0.996 7、0.977 7和0.998 5,且经过拟二级动力学方程计算得到qe分别为0.861 0、0.283 5、3.647 0 和15.600 6 mg/g。说明活性炭对水中NH4+-N、TP、TN和COD的吸附符合拟二级动力学,而且由实验获得的平衡吸附容量与拟二级动力学模型计算得到的理论吸附量吻合得很好,进一步说明活性炭对水中NH4+-N、TP、TN和COD的吸附存在化学吸附。王芳[24]在改性活性炭吸附污水氨氮性能的研究中表明污水中氨氮的吸附动力学更符合拟二级动力学方程。
-
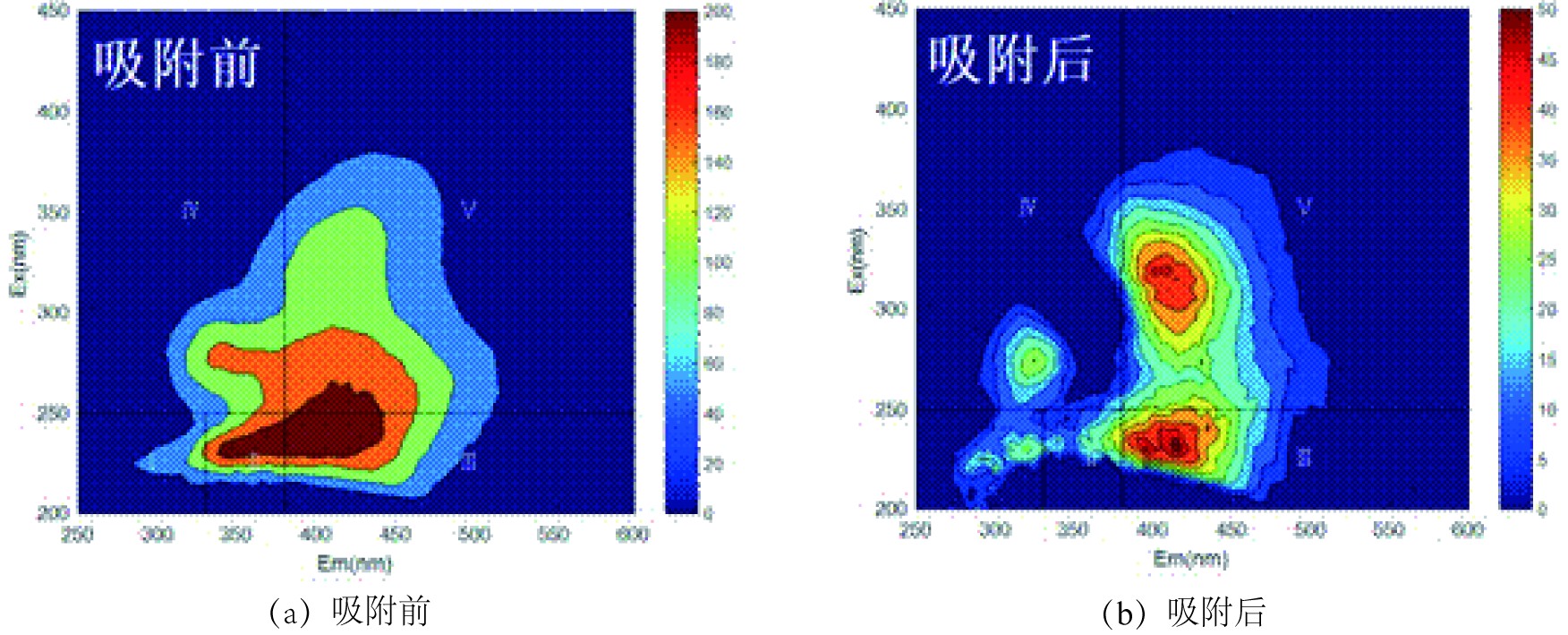
污水厂尾水经活性炭吸附前后的三维荧光光谱,见图11,根据荧光区域积分(FRI)的方法[25]将其划分为5个区进行分析。
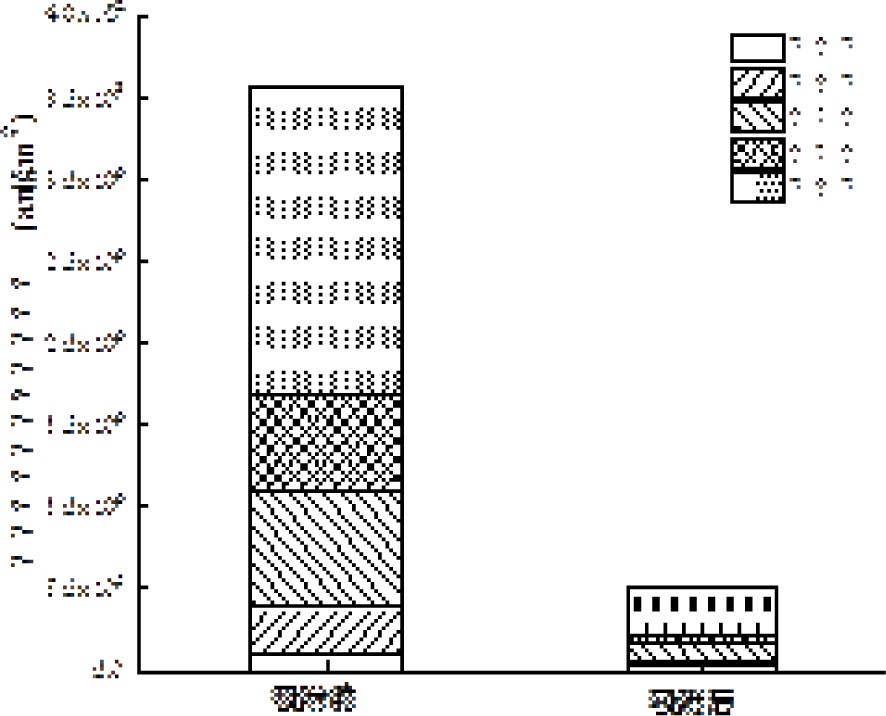
图11可知,污水厂尾水中存在的主要溶解性有机物是酪氨酸类蛋白质、色氨酸类蛋白质、富里酸类物质、溶解性微生物代谢产物和腐殖酸类物质[26]。经吸附后,溶解性有机物的最大荧光强度由200降低至50,表明有机物在吸附过程中得到了有效去除。为了更深层次探究溶解性有机物的荧光特性,运用荧光光谱FRI对溶解性有机物的三维荧光光谱进行定量分析。污水厂尾水经活性炭吸附前后的总积分标准体积,见图12。
图12可知,活性炭吸附后的溶解性有机物荧光强度低于吸附前的溶解性有机物荧光强度。各荧光组分占比,见图13。
图13可知,吸附前5个荧光组分积分标准体积在总积分标准体积中的占比均值从大到小依次排序为:腐殖酸类物质(52.70%)﹥溶解性微生物代谢产物(16.40%)﹥富里酸类物质(19.96%)﹥色氨酸类蛋白质(8.00%)﹥酪氨酸类蛋白质(2.95%)。吸附后5个荧光组分积分标准体积在总积分标准体积中的占比均值从大到小依次排序为:腐殖酸类物质(58.05%)﹥富里酸类物质(22.19%)﹥溶解性微生物代谢产物(10.73%)﹥酪氨酸类蛋白质 (4.65%)﹥色氨酸类蛋白质(4.38%)。
-
(1)通过单因素实验得到活性炭吸附最佳实验条件为:温度25 ℃,活性炭投加量1.5 g/L,吸附时间80 min,pH为7。在此条件下,COD、NH4+-N、TN和TP出水浓度分别为5 、0.03 、9.37和0.03 mg/L,NH4+-N、TP、TN和COD去除率分别为98.01%、94.04%、32.40%和82.14%,出水浓度满足《城市污水再生利用景观环境用水水质:GB/T 18921—2019》标准。
(2)采用拟一级动力学及拟二级动力学方程对活性炭吸附NH4+-N、TP、TN和COD过程进行分析,动力学研究表明,Langergren拟二级动力学方程能较好地反映出该吸附的动力学过程。此外,拟二级动力学计算得到的qe更接近于实验得到的qe,说明活性炭对水中NH4+-N、TP、TN和COD的吸附存在化学吸附。
(3)吸附法处理污水厂二沉池出水,出水指标均达到再生水回用于景观用水水质标准的要求,并且降低了经济成本带来良好的社会效益和经济效益,说明吸附法可以满足作为景观回用的技术。
(4)三维荧光光谱研究表明,经吸附后溶解性有机物的最大荧光强度显著降低,活性炭吸附后的溶解性有机物荧光强度低于吸附前的溶解性有机物荧光强度,尾水中溶解性有机物主要为腐殖酸类物质。
吸附法处理污水厂尾水的性能以及再生利用简析
Study on the effect and characteristics of tailwater recycling of sewage treatment plant by adsorption method
-
摘要: 水资源是制约经济发展的重要因素,再生水循环利用是解决这一问题的主要途径,再生水用于景观用水时,氨氮、总磷、总氮及化学需氧量指标常常成为限制因素。以某生活污水厂二沉池出水为处理对象,研究了活性炭投加量、吸附时间、初始pH和温度对吸附效率的影响,分别采用拟一级动力学方程和拟二级动力学方程对NH4+-N、TP、TN和COD吸附实验的动力学进行研究,通过采用三维荧光光谱法及荧光区域积分分析 (FRI)对二沉池出水进行定量分析,考察活性炭吸附效果。结果表明:在温度25 ℃,活性炭投加量1.5 g/L,吸附时间80 min,pH=7条件下,NH4+-N、TP、TN和COD去除率分别为98.01%、94.04%、32.40%和82.14%,处理效果良好,技术经济可行,满足再生水景观回用标准。动力学研究表明,Langergren准二级动力学方程能较好地反映出活性炭吸附NH4+-N、TP、TN和COD的动力学过程,吸附存在化学吸附。三维荧光光谱法研究表明,活性炭吸附对二沉池出水中有机物具有较好的降解作用。Abstract: Water resources is an important factor restricting economic development, reclaimed water recycling is the main way to solve this problem, reclaimed water used for landscape water, ammonia nitrogen, total phosphorus, total nitrogen and chemical oxygen demand indicators often become limiting factors. Taking the effluent of the second sedimentation tank of a domestic sewage plant as the treatment object, the effects of activated carbon dosage, adsorption time, initial pH value and temperature on the adsorption efficiency were studied, and the kinetics of NH4+-N, TP, TN and COD adsorption experiments were studied by quasi-first-order kinetic equations and quasi-second-order kinetic equations, and the effluent of the secondary sedimentation tank was quantitatively analyzed by three-dimensional fluorescence spectroscopy and fluorescence region integral analysis (FRI) to investigate the adsorption effect of activated carbon. The results showed that the removal rates of NH4+-N, TP, TN and COD were 98.01%, 94.04%, 32.40% and 82.14%, respectively, at a temperature of 25 °C, the dosage of activated carbon was 1.5 g/L, the adsorption time was 80 min, and the pH=7 conditions were 98.01%, 94.04%, 32.40% and 82.14%, respectively, with good treatment effect, technical and economic feasibility, and met the standards of reclaimed water landscape reuse. Kinetic studies show that the quasi-secondary kinetic equation of Langergren can better reflect the kinetic process of activated carbon adsorption of NH4+-N, TP, TN and COD, and the adsorption is chemically adsorbed. The three-dimensional fluorescence spectroscopy study showed that the adsorption of activated carbon had a good degradation effect on the organic matter in the effluent of the second sedimentation tank.
-
Key words:
- reclaimed water /
- activated carbon /
- adsorption /
- single factor /
- domestic sewage treatment plant
-
生物完整性指数(index of biological integrity, IBI)通过从候选参数中选取对人类干扰较为敏感的核心参数,构建评估体系评估河流生态健康[1]。最早是由KARR[2]以鱼类作为指示生物而提出的,而后研究对象逐步发展至浮游生物[3-5]、藻类[6-7]和大型底栖动物[8-9]等类群。作为河流中稳定的生物类群,大型底栖动物具有种类丰富、生命周期长、迁移能力弱、方便采集,且不同种类对环境变化敏感性呈现出较大差异性等特点[7-10],能够较为准确地表征河流生态环境状况和人类活动对河流水体的扰动程度,对河流健康具有较为精准的诊断作用,是构建IBI的理想对象。
KERANS et al[11]最先利用大型底栖动物完整性指数(benthic index of biological integrity, B-IBI)进行了河流水生态健康评价,而后该指数在全球范围内得到了广泛应用,并取得了良好的河流健康诊断效果[12-15]。我国在20世纪80年代就已应用大型底栖动物评价河流水质[16],王备新等[9]首次在国内应用B-IBI评价了黄山地区的溪流,随后B-IBI在我国地表水水体评价中得到广泛应用。然而,现有研究多为我国南部地区河流湖泊,有关西北干旱区河流研究较少,且不同地区河流底质和环境状况各异,B-IBI体系也并不相同,因此,构建适用于西北地区干旱半干旱区河流B-IBI体系是非常必要的。
清水河是宁夏境内黄河最长的一级支流,流域水资源极度匮乏,河流水体矿化度高、天然水质差、含沙量大,天然来水年内分配不均且年际变化悬殊,具有干旱半干旱地区河流的典型特征[17]。关于清水河这类干旱半干旱区域河流的水生生物研究相对较少,对底栖动物群落结构组成、健康状况评估及其与水环境要素的关系研究尚未见报道。故本研究以大型底栖动物为指示生物,以水质较好及Shannon-Wiener多样性指数较大、较少人类活动的点作为参考点,构建B-IBI体系对清水河健康状况进行评估,并分析对B-IBI有显著影响的环境因子,对于改善清水河河流健康状况具有指导作用,也为开展干旱半干旱区域河流健康评估提供一定的借鉴。
1. 研究区域与方法
1.1 区域概况
清水河位于宁夏中南部地区,地势南高北低,全长320 km,流域面积14 481 km2[18-19]。受温带大陆性气候影响,流域干旱少雨,雨季集中在7~10月,降雨时空不均匀,水资源十分短缺,水土流失现象严重,具有半干旱、干旱区河流特征,是宁夏生态环境最为脆弱的区域之一[20]。
1.2 样品采集处理与分析
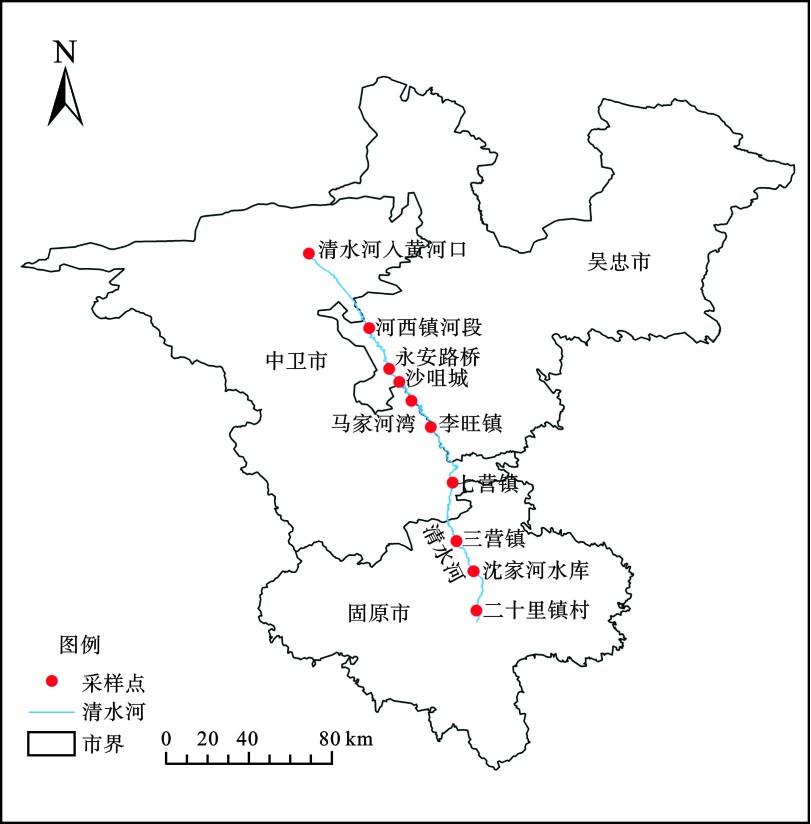
于2020年7月开展现场采样及监测工作,沿清水河干流在已有6个国控、区控监测断面(点位)的基础上(其中三营镇、入黄河口为国控,其余为区控)另根据清水河从南到北依次穿越宁夏南部黄土丘陵区、中部干旱风沙区和北部引黄灌溉区等各区域的实际情况,重点考虑采样点的交通可达性、生境的典型性等合理增设4个采样点,共设置10个采样点,所布设的采样点基本可代表清水河干流的整体特征。采样点位置,见图1。
水温、电导率和pH采用多功能水质分析仪现场监测,溶解氧采用便携式溶解氧分析仪现场监测,COD、BOD5、氨氮和总磷等水质指标根据《水和废水监测分析方法》在实验室测定。
采用1/16 m2改良彼得逊采泥器和手抄网(直径40 cm)进行大型底栖无脊椎动物的采集,用底栖筛(420 μm)淘洗后挑拣出放入50 mL标本瓶中,加入10%甲醛溶液保存,在实验室用显微镜镜检将物种鉴定至最低的分类单元并计数,最后用滤纸吸干水分,在万分之一天平上称量获得生物量数据。
1.3 B-IBI评估体系构建
1.3.1 确定参考点与受损点
参考点是指没有遭受人类活动扰动或者扰动较小的点位,是利用IBI进行河流健康评价的基准。因目前除部分湿地保护区河流外,其他河流均或多或少受到人类活动干扰,不存在严格意义上的参考点,因此本研究结合已有参照点选取的方法和实际情况,确立了参照点的选取标准[21-22]:水质综合评价在Ⅲ类及以上,Shannon-Wiener多样性指数>2(以2为底);极少人类活动、两岸500 m内无农田(定性观察)。
1.3.2 筛选评估参数
根据以往的研究[1,23]和清水河实际,本研究选取了能反映丰富度与多样性、群落结构组成、耐污能力、功能摄食类群与生活型等类型的20个参数(即表1的M1~M20)作为候选参数,采用如下方法筛选出核心参数:(1)变异度分析:计算参考点评估参数的平均值、标准差、最大值、最小值、极差、25%分位数和75%分位数,保留变异度较小的参数。(2)判别能力分析-利用箱线图对比参考点和受损点在25%~75%分位数范围内箱体的重叠程度,对生物判别能力(interquartile range, IQ)赋值,保留部分重叠但中位数均在对方箱体之外(IQ=2)和箱体无重叠(IQ=3)的参数。(3)冗余度分析-采用Spearman相关性分析,ρ>0.8时表明2个参数有显著相关性,此时选其中1个即能表示2个参数间所包含的几乎全部的信息,优先选取反映信息较多的参数。
表 1 候选参数及其对干扰响应的方向Table 1. Candidate metrics and their response to disturbance参数编号 参数类型 参数 对干扰增大的响应 M1 多样性和丰富性 总分类单元数 减小 M2 EPT分类单元数 减小 M3 蜉蝣目分类单元数 减小 M4 鞘翅目分类单元数 减小 M5M6 半翅目分类单元数Shannon-Wiener多样性指数 减小减小 M7 群落结构组成 EPT个体数百分比 减小 M8M9 蜉蝣目个体数百分比半翅目个体数百分比 减小减小 M10 最优类群个体数百分比 减小 M11 耐污能力 敏感类群分类单元数 减小 M12 耐污类群分类单元数 增大 M13 Hilsenhoff生物指数(HBI) 增大 M14 大型无脊椎动物敏感类群评估指数(BMWP指数) 减小 M15 ASPT指数 减小 M16 科级耐污指数(FBI) 增大 M17 功能摄食类群与生活型 粘附者分类单元数 减小 M18 粘附者个体数百分比 减小 M19 滤食者个体数百分比 增大 M20 刮食者个体数百分比 减小 1.3.3 计算B-IBI指数
选取比值法计算B-IBI的值[23]。对于随着干扰增大而减小的参数,应按由低至高排序的以95%分位数作为最佳期望值,该类参数的分值为参数实际值与最佳期望值的比值;对于随着干扰增大而增大的参数,应以由低至高排序的5%分位数作为最佳期望值,该类参数的分值为最大值与实际值、最佳期望值差值的比值。最终计算各类参数和,即为各断面B-IBI指数值[24]。
1.3.4 建立评价标准
得到各样点的B-IBI值后,选取所有点位B-IBI分值的95%分位值作为最佳期望值,然后利用四分法确定河流健康等级划定标准[21],按照《河流水生态环境质量监测与评价技术指南(征求意见稿)(2020)》将河流分为5个健康等级:非常健康、健康、亚健康、不健康和病态,分级标准,见表2。
表 2 河流健康等级划定标准Table 2. River health assessment grading on B-IBI健康等级 B-IBI得分 病态 (0~1.25] 不健康 (1.25~1.83] 亚健康 (1.83~1.94] 健康 (1.94~2.83] 非常健康 (>2.83) 2. 结果与分析
2.1 大型底栖动物群落结构
本次调查共采集到大型底栖动物42个分类单元,隶属于3门4纲11目32科,其中昆虫纲6目27科35种属,占物种总比例83.33%,软甲纲2目2科3种属,占比7.14%,腹足纲1目2科3种属,占比7.14%,蛭纲1种属,占比2.38%。
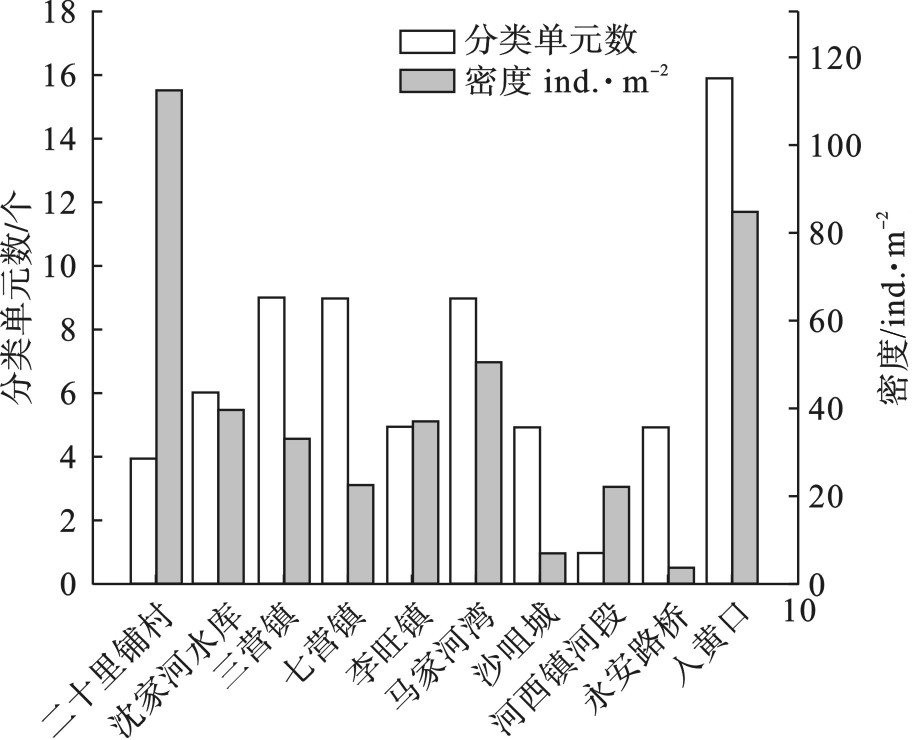
调查期间,各样点采集到的大型底栖动物分类单元数差别较大,其中三营镇、七营镇、马家河湾和入黄河口物种较为丰富。清水河底栖动物密度为4~112.5 ind./m2,平均密度41.45 ind./m2;其中水生昆虫类密度最高,为375 ind./m2,占总密度的90.5%,其次为软甲类,占比8.1%;蛭纲和腹足纲密度较低,分别为2 ind./m2和4 ind./m2。见图2。
清水河各点位底栖动物分布,见图3。
图3可知,清水河水生昆虫种类比例较多,种群出现频率前3名分别是划蝽科、龙虱科和水龟甲科,出现频率分别为90%、60%和50%,均为水生昆虫。划蝽科为第一优势种,耐污性较强,而敏感性物种,如蜉蝣目的扁蜉科仅出现在生态较好的清水河入黄口,其他采样点位没有发现。
2.2 B-IBI指数构建
2.2.1 参照点与受损点
通过对各监测断面水质监测和实地调查,三营镇、七营镇、沙咀城和清水河干流入黄河口4个点位符合全部参考点选取的标准要求,确定为参考点,其余断面作为受损点。
2.2.2 核心参数的确定
(1) 变异度分析。对20个参数在参考点中的平均值、标准差、最大值、最小值、极差、25%分位数和75%分位数进行比较,见表3,剔除数值范围变动太小或太大的参数,它们不适合用来建立B-IBI评价体系。
表 3 候选参数变异度分析结果Table 3. Variation results of candidate metrics参数编号 平均值 标准差 最小值 最大值 极差 25%分位数 75%分位数 M1 9.75 3.96 5.00 16.00 11.00 8.00 10.75 M2 0.50 0.87 0.00 2.00 2.00 0.00 0.50 M3 0.25 0.43 0.00 1.00 1.00 0.00 0.25 M4 2.50 0.87 2.00 4.00 2.00 2.00 2.50 M5 2.75 1.92 0.00 5.00 5.00 1.50 4.25 M6 2.42 0.18 2.23 2.60 0.37 2.24 2.60 M7 2.06% 3.57% 0.00% 8.24% 8.24% 0.00% 2.06% M8 1.47% 2.55% 0.00% 5.88% 5.88% 0.00% 1.47% M9 36.15% 19.09% 14.29% 60.87% 46.58% 19.48% 51.39% M10 33.72% 17.94% 14.29% 56.52% 42.24% 17.21% 48.54% M11 1.25 1.09 0.00 3.00 3.00 0.75 1.50 M12 8.50 3.20 4.00 13.00 9.00 7.00 10.00 M13 4.95 0.50 4.34 5.45 1.11 4.51 5.44 M14 40.00 16.81 28.00 69.00 41.00 30.25 41.25 M15 4.66 0.59 4.00 5.60 1.60 4.32 4.85 M16 4.99 0.52 4.36 5.52 1.16 4.52 5.49 M17 0.75 0.83 0.00 2.00 2.00 0.00 1.25 M18 7.26% 10.23% 0.00% 24.71% 24.71% 0.00% 9.44% M19 5.49% 4.83% 0.00% 13.04% 13.04% 2.27% 7.67% M20 6.56% 5.08% 0.00% 14.29% 14.29% 4.41% 8.12% 首先删除最大值过小的参数EPT分类单元数M2、蜉蝣目分类单元数M3。鞘翅目分类单元数M4、Shannon-Wiener多样性指数M6、EPT个体数百分比M7、蜉蝣目个体数百分比M8、半翅目个体数百分比M9、敏感类群分类单元数M11和ASPT指数M15,这些随干扰增加而减小的参数,其25%分位数、75%分位数变化很小,说明随着干扰增大,参数值基本不变,不能有效反映干扰对河流的影响,应剔除;Hilsenhoff生物指数(HBI)M13、科级耐污指数(FBI)M16随干扰增加而增大,随干扰增大,其25%分位数、75%分位数基本不变,故也应剔除。剩9个参数进入下一步判别能力分析。
(2) 判别能力分析。剩余9个候选参数参考点和受损点的箱体IQ重叠情况,见图4。
图4可知,M1、M5、M10、M12和M20等5个参数符合IQ≥2的情况,这说明参数在受损点和参考点之间差别显著,保留5个参数进行冗余度分析。
(3) 冗余度分析。对剩余参数开展Spearman相关分析的结果,见表4。M1和M12相关性显著,M1能反映更多信息,故剔除M12,保留M1。
表 4 候选参数相关性分析结果Table 4. Spearman correlation analysis of candidate metrics参数 M1 M5 M10 M12 M20 M1 1.00 M5 0.77 1.00 M10 −0.18 −0.41 1.00 M12 0.97** 0.77 −0.24 1.00 M20 0.48 0.20 −0.48 0.56 1.00 注:**表示p<0.01。 2.2.3 B-IBI指数的计算
根据4个参数对干扰增大的响应,计算它们在所有样点中的95%分位数或5%分位数的值和最佳期望值,见表5,根据比值法计算采样点4个参数的分值,加和即得各断面的B-IBI值,见表6。
表 5 核心参数分位数计算结果Table 5. Quantile calculation results of core metrics参数编号 对干扰的响应 最大值 5%分位数 95%分位数 最佳期望值 M1 减小 16.00 2.35 12.85 12.85 M5 减小 5.00 0.00 4.55 4.55 M10 减小 100.00% 0.06 0.93 0.93 M20 减小 14.29% 0.00 0.11 0.11 表 6 各断面B-IBI值和断面评估结果Table 6. Grade for B-IBI of river health assessment断面 断面类型 B-IBI 健康度 二十里铺村 受损点 0.53 病态 沈家河水库 受损点 1.77 不健康 三营镇 参考点 1.91 亚健康 七营镇 参考点 2.41 健康 李旺镇 受损点 1.29 不健康 马家河湾 受损点 2.03 健康 沙咀城 参考点 1.89 亚健康 永安路桥 受损点 1.07 病态 河西镇河段 受损点 1.16 病态 清水河干流入黄河口 参考点 3.17 非常健康 2.3 B-IBI指数评价
以参考样点B-IBI值由高到低排序,计算所有点位95%分位数作为B-IBI的最佳期望值为2.83,按照等级划定标准表进行赋分,判断健康度,健康评估,见表6。
2.4 B-IBI指数与环境因子分析
对采样点B-IBI和环境因子进行Kolmogorov-Smirnov正态性检验,结果表明两者均满足正态分布。采用Pearson相关分析分析影响B-IBI的环境因子,见表7,清水河B-IBI指数仅与氟化物具有显著的相关性,而与pH、COD、流速、水温、电导率、透明度、溶解氧、氨氮和总磷等相关性并不显著,表明影响清水河B-IBI的主要环境因子为氟化物浓度。
表 7 B-IBI指数与水质指标Pearson相关关系Table 7. Pearson correlation analysis between the B-IBI and environmental factors水质指标 相关系数 水质指标 相关系数 pH −0.30 透明度 −0.40 COD −0.62 溶解氧 0.05 流速 0.34 氨氮 −0.41 水温 −0.25 氟化物 −0.72** 电导率 0.04 总磷 −0.12 注:**表示p<0.01,*表示p<0.05。 3. 讨论
3.1 生物指数筛选与清水河健康评价
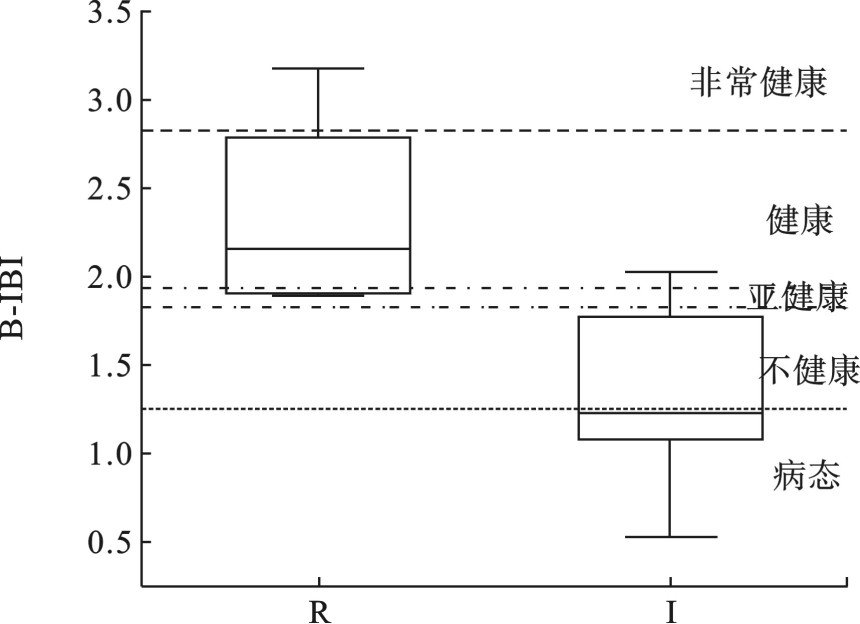
不同地区河流底质和环境状况不同,大型底栖动物群落组成也不同[25-26],因此构建的B-IBI指标体系也各异。本研究通过箱线图及冗余度分析,选择了总分类单元数、半翅目分类单元数、最优类群个体数百分比和刮食者个体数百分比共4个核心参数构建了宁夏清水河B-IBI指标体系。清水河B-IBI值的箱线图分布,见图5,校验结果显示,参考点和受损点箱体无重叠,参照点的箱体基本落在健康、非常健康级别,受损点箱体落在亚健康、不健康和病态级别,这表明参照点的选取是有效的,本研究所建立的B-IBI评价体系适用于宁夏清水河健康评价。
从参考点的健康状态来看,1处非常健康,1处健康,另外2处为亚健康,在清水河评估的10个点位上,均为较好的状态。受损点马家河湾处为健康状态,该点位为健康状态主要是受底栖动物总分类单元数、最优类群个体数这2类核心参数的数值的影响,也表明应用底栖动物完整性指数评估清水河健康状态时,在参数的选取上还需要进一步地深入研究和优化。
通过对清水河进行评价,结果显示,宁夏清水河处于非常健康、健康、亚健康、不健康和病态水平的点位分别占总点位的10%、20%、20%、20%和30%。健康状况为“病态”的点位在二十里铺、永安路桥和河西镇河段,可能与二十里铺水质较差、永安路桥上游处有农田退水,水质变差有关。健康状况为“亚健康”“不健康”的点主要位于支流汇入口,为宁夏中部干旱风沙区,主要土地类型为裸地,存在的问题主要是自然栖息地破坏严重,植被退化,受人类干扰胁迫较大。健康水平较高的点位主要位于清水河北部入黄口,河流连通性较好,水质及生境较好,河道底质多为卵石和砾石,有利于水生昆虫附着生存。
3.2 B-IBI指数影响因子分析
将B-IBI指数与环境因子进行Pearson相关性分析,结果表明,B-IBI与氟化物关联最强,而同时氟化物也是限制清水河受损点水质类别的主要参数。文献[27]研究表明,氟化物具有高度毒性,会对大型底栖动物群落组成和分布产生影响。通常水中氟化物浓度越高,表示人为扰动越强烈,B-IBI的分值就越低。因此,有效降低河流氟化物浓度,是当前恢复清水河生态健康的重点。
清水河部分区域地层岩石以高氟岩石为主,地下水流经时通过侵蚀作用使氟化物进入水体,后地下水补充地表水时就会使清水河含氟量较高[18];另一方面,清水河流域地处干旱半干旱地带,水体蒸发作用强烈,氟化物被留在土壤中,加之人为活动如河岸带土地利用,农田开垦等导致清水河下垫面降低,河岸带植被稀疏,水土流失严重,大量氟化物进入水体,影响河流水质,并对水生生物产生不利影响[18]。受支流汇入产生的叠加效应,流域气候、地貌等因素,干流各采样点氟化物浓度不同[18],对大型底栖动物影响也不同。氟化物可以通过摄食或皮肤接触进入底栖动物体内,并在其体内蓄积,使底栖动物生长缓慢、繁殖率降低,死亡率大大增加[27]。可见,进行水土流失控制与治理,优化河岸带状况能有效改善清水河底栖动物群落结构,维护和修复清水河生态健康。
-
表 1 实验用水水质参数
Table 1. Experimental water quality parameters mg·L−1
NH4+-N TN TP COD ≤5 ≤10 ≤0.5 ≤50 表 2 吸附动力学拟合结果
Table 2. Adsorption kinetics fitting results
水质指标 C0/mg·L−1 qe,exp/mg·g−11 拟一阶动力学模型 拟二阶动力学模型 qe,cal/mg·g−11 R2 k1/min−11 qe,cal/mg·g−11 R2 k2/g·(mg·min)−11 NH4+-N 1.41 0.833 3 0.426 3 0.830 1 0.032 7 0.861 0 0.998 7 0.196 9 TP 0.49 0.266 7 0.431 1 0.784 3 0.052 9 0.283 5 0.996 7 0.296 2 TN 14.75 3.226 7 8.167 8 0.864 6 0.075 0 3.647 0 0.977 7 0.014 4 COD 28.00 14.666 7 45.768 7 0.779 7 0.078 7 15.600 6 0.998 5 0.006 4 -
[1] 李一, 刘宏权, 陈任强, 等. 再生水灌溉对作物和土壤的影响[J]. 灌溉排水学报, 2022, 41(增1): 26 − 33. doi: 10.13522/j.cnki.ggps.2022084 [2] CAO Y Q, FU X P, WANG H, et al. Discussion on ecological system management of urban reclaimed water environment-a case study of Xihua Park in Kunming[J]. IOP conference series:Earth and environmental science, 2021, 772(1): 12 − 86. [3] 张庆康, 郝瑞霞, 刘峰, 等. 不同再生水处理工艺出水水质回用途径适应性分析[J]. 环境工程学报, 2013, 7(1): 91 − 96. [4] 宋永会, 郑丙辉, 刘佑华, 等. 水生植物法再生景观回用水水质稳定技术研究[J]. 环境科学研究, 2007(1): 80 − 84. doi: 10.3321/j.issn:1001-6929.2007.01.015 [5] 吴江伟, 楚金喜, 吕丹. 基于混凝沉淀工艺的城市污水处理厂尾水深度脱色技术研究[J]. 工业安全与环保, 2021, 47(11): 99 − 102. doi: 10.3969/j.issn.1001-425X.2021.11.023 [6] WANG Y X, DUAN J M, LIU S X, et al. Removal of As(III) and As(V) by ferric salts coagulation – Implications of particle size and zeta potential of precipitates[J]. Separation & purification technology, 2014, 135: 64 − 71. [7] 杨沙沙. 城市污水处理厂污水深度处理工艺研究[D]. 济南: 山东大学, 2012. [8] LIU L, LI C, LAI R T, et al. Perturbation and strengthening effects of DOM on the biochar adsorption pathway[J]. Ecotoxicology and environmental safety, 2022, 245: 113 − 114. [9] 郑晓英, 王俭龙, 李鑫玮, 等. 臭氧氧化深度处理二级处理出水的研究[J]. 中国环境科学, 2014, 34(05): 1159 − 1165. [10] WANG Y F, WANG N, LI M, et al. Potassium ferrate enhances ozone treatment of pharmaceutical wastewaters: Oxidation and catalysis[J]. Journal of water process engineering, 2022, 49: 55 − 103. [11] 王翔, 朱召军, 尹敏敏, 等. 组合人工湿地用于城市污水处理厂尾水深度处理[J]. 中国给水排水, 2020, 36(06): 97 − 101. doi: 10.19853/j.zgjsps.1000-4602.2020.06.019 [12] Lei Y, WAGNER T, RIGNAARTS H, et al. The removal of micropollutants from treated effluent by batch-operated pilot-scale constructed wetlands[J]. Water research, 2023, 230: 119 − 494. [13] 韩玉珠, 马青兰. 混凝沉淀法污水深度处理条件优化[J]. 净水技术, 2011, 30(01): 42 − 44. doi: 10.3969/j.issn.1009-0177.2011.01.011 [14] 李夏青, 赵新华. 臭氧氧化法用于再生水回用的研究[J]. 安徽农业科学, 2011, 39(22): 13681 − 13682. doi: 10.3969/j.issn.0517-6611.2011.22.152 [15] 关永年, 刘洪波, 黄剑虹, 等. 污水处理厂二级出水粉末活性炭深度处理试验[J]. 净水技术, 2022, 41(04): 61 − 65. doi: 10.15890/j.cnki.jsjs.2022.04.010 [16] 魏俊起, 颜小星. 粉末活性炭去除污水处理厂二沉池出水中难降解COD的试验研究[J]. 城市住宅, 2015(08): 106 − 108. [17] 刘寒冰, 杨兵, 薛南冬. 酸碱改性活性炭及其对甲苯吸附的影响[J]. 环境科学, 2016, 37(09): 3670 − 3678. doi: 10.13227/j.hjkx.2016.09.051 [18] 唐安琪. 吸附及臭氧氧化联用处理煤化工废水生化出水试验研究[D]. 哈尔滨: 哈尔滨工业大学, 2014. [19] 张存芳, 王鹏程, 吕斯濠, 等. 活性炭吸附法去除废水COD的研究[J]. 广东化工, 2018, 45(02): 29 − 30. doi: 10.3969/j.issn.1007-1865.2018.02.012 [20] 李玉, 刘俊, 陆英, 等. 改性活性炭吸附处理含铅废水的研究[J]. 广东化工, 2022, 49(01): 147 − 149. doi: 10.3969/j.issn.1007-1865.2022.01.048 [21] 殷玉蓉, 凌琪, 伍昌年. 改性活性炭对污水厂尾水深度处理的实验研究[J]. 中国西部科技, 2013, 12(03): 19 − 21. [22] 马留可, 詹福如. 活性炭对水中亚甲基蓝的吸附性能研究[J]. 化学工程, 2016, 44(01): 28 − 32. doi: 10.3969/j.issn.1005-9954.2016.01.007 [23] 周强, 段钰锋, 冒咏秋, 等. 活性炭汞吸附动力学及吸附机制研究[J]. 中国电机工程学报, 2013, 33(29): 10 − 17. doi: 10.13334/j.0258-8013.pcsee.2013.29.002 [24] 王芳. 改性活性炭吸附污水中氨氮的性能[J]. 应用化工, 2015, 44(05): 874 − 877. doi: 10.16581/j.cnki.issn1671-3206.2015.05.025 [25] Chen W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter[J]. Environmental science & technology, 2003, 37(24): 5701 − 5710. [26] He X S, Xi B D, Wei Z M, et al. Fluorescence excitation-emission matrix spectroscopy with regional integration analysis for characterizing composition and transformation of dissolved organic matter in landfill leachates[J]. Journal of hazardous materials, 2011, 190(1-3): 293 − 299. doi: 10.1016/j.jhazmat.2011.03.047 -




 下载:
下载: