-
氟是一种人体必须的微量元素,适量的氟有利于预防骨质疏松和龋齿,但过量的摄入会导致骨髓畸形和脑损伤等疾病。在自然界中氟元素会在风化作用下进入地下水,人类活动如煤矿、玻璃陶瓷、半导体制造和电镀等工业,也会在生产制造过程中排出大量高浓度的含氟废水,之后与人类活动产生的有机污染物混合后,会形成较难处理的氟离子和有机物混合废水。针对此类废水,常用的处理方法有混凝法[1-2]、吸附法[3-4]、离子交换法[5-6]、电渗析法、反渗透膜法[7]和电絮凝法[8]等。吸附法具有反应速率快和工艺简单的优势,但仅在污染物浓度较低时有效,而且吸附剂的处理和再生成本较高[9]。离子交换法可以针对性地去除水中的氟离子,但无法去除有机物,且存在树脂再生困难和出现2次污染问题[10];膜处理法可以有效去除水中的氟离子和有机物,但其由于对设备要求高、滤膜易堵塞以及2次浓水处理困难等问题,在实际应用中会受到限制[11]。
以氯化铝(AlCl3)为代表的传统铝系混凝剂凭借着处理效果稳定和价格低廉的优点[12],广泛应用于中小型水处理措施中。但其存在絮体体积小且结构疏松、低温下混凝效果差、药剂投入量大和余铝不可控等问题,极少应用在大规模工程中[13]。高聚十三铝(Al13)是一种纳米级尺寸和高正电荷密度的溶胶大分子新型混凝剂,有极佳的混凝水解形态[14-16]。相比于传统的铝盐混凝剂,Al13经过预水解生成的产物更倾向于保持其化学特性,因此有较高的水解稳定性,并且其高正电荷密度的特点,更有利于接近和吸附污染物。此外,氢键和离子交换机制也进一步保证了高效的混凝效果。腐殖酸(HA)是自然界中最常见的有机质[17-18],由动植物遗骸经过微生物的分解和转化形成,其分子质量分布在800~6 000 Da,通常作为模型物质替代天然有机物开展研究。研究表明,AlCl3和Al13作为铝系混凝剂对水中的氟离子具有高效的混凝去除能力[19]。并且借助电中和、网捕卷扫等机理铝系混凝剂也具有一定的去除有机物的能力[20]。然而,不同铝系混凝剂在处理氟离子和有机物混合废水时,均会面临混凝剂性能下降,氟离子去除效率明显下降的问题。
为揭示不同铝系混凝剂在处理氟离子和有机物混合废水时的作用机理,解释混凝剂在处理氟离子和有机物混合废水时,氟离子去除效率下降的现象,本研究制备不同HA浓度的含氟废水作为实验水体进行实验[21-22],通过对比不同形态铝混凝剂对混合废水中氟离子的去除效果,探讨其内在的作用机理,以期对氟离子与有机物混合废水处理或供水厂工艺选择提供参考。
-
六水合氯化铝(AlCl3·6H2O)、氢氧化钠(NaOH)、盐酸(HCl)、氟化钠(NaF)、硝酸钠(NaNO3)、二水合氯化钡(BaCl2·2H2O)、硫酸钠(Na2SO4)和乙酸钠(CH3COONa)分析纯,均购自国药集团化学药剂有限公司。二水合柠檬酸三钠(Na3C6H5O7·2H2O)分析纯、HA ≥ 90%购自阿拉丁试剂公司,Ferron试剂分析纯,购自Sigma-Aldrich试剂公司。主要仪器有电喷雾四极杆飞行时间质谱(ESI-TOF-MS)(ACQUITY UPLC/Xevo G2 Q TOF, Waters, USA)、紫外可见分光光度计(UH5300, ShimadZu, Japan)、六联搅拌仪(MY 3000-2J/4J,梅宇,中国)、Zeta电位分析仪(ZetasiZer Nano ZS90, Malvern, UK)、离子选择电极(PHS-3C,雷磁,中国)、电感耦合等离子体发射光谱法(ICP-OES)(ICPE-9800, ShimadZu, Japan)、利用傅立叶变换红外光谱仪(FTIR)(Nicolet 8700, ThermoFisher, USA)、X射线光电子能谱仪(XPS)(ESCALAB250Xi,ThermoFisher, USA)、总有机碳分析仪(ShimadZu,TOC-L,日本)。
-
实验中AlCl3溶液由药品直接配制。Al13由碱化度2.2的聚合氯化铝溶液,利用硫酸钠和氯化钡沉淀置换法提纯得到[23],在确定产物纯度后,利用超纯水稀释质量浓度至2.5 g·L−1。2种混凝剂均为现用现制,浓度以Al的质量计算。将Al13和AlCl3溶液稀释至16.2 mg·L−1后,利用Al-Ferron逐时络合比色法[24]并结合紫外可见分光光度计测定2种混凝剂中Al的形态(表1)。结果表明,AlCl3中主要的铝形态为Ala,而Al13中主要铝形态为Alb。
-
1)模拟水样配置。取10.0 g HA粉末分散于1 L超纯水中,加入0.4 g NaOH不断搅拌至HA溶解。持续搅拌5 h后,用0.45 μm滤膜过滤。利用总有机碳分析仪确定储备液的溶解性有机碳(DOC) 质量浓度,为2 791.7 mg·L−1。定量加入去离子水,将储备液稀释至DOC为2 000.0 mg·L−1,即可得HA储备液。将NaF药品置于110 ℃的烘箱中干燥4 h后,精确称量并配置成含氟离子浓度为1.0 g·L−1的NaF储备液。在超纯水中引入NaF储备液,将氟离子质量浓度确定为8.0 mg·L−1。再加入不同浓度HA储备液,制成6种模拟水样,DOC值分别为0、5、10、20、35和50 mg·L−1。上述6种水样中,均需加入10.0 mmol·L−1 NaNO3和4.0 mmol·L−1 NaHCO3溶液以调节离子强度和碱度。最终,利用0.1 mol·L−1的HCl和NaOH溶液调节水样pH至8.0。
2)混凝实验。室温下使用搅拌仪进行混凝实验。在添加混凝剂之前,首先将500 mL模拟水样以250 rpm的速度搅拌30 s。投加药剂后,以200 r·min−1的转速搅拌90 s充分混合。测试样品Zeta电位3次并取平均值。降低搅拌速度至40 r·min−1,慢搅10 min令絮体生长。沉降30 min后,利用0.45 μm滤膜对上清液进行过滤,对渗出液中氟化物质量浓度、DOC值和余铝质量浓度进行测定。
3)动态吸附实验。通过动态吸附实验探究氟离子、有机物和Al13三者之间的作用顺序。在无氟HA溶液中,投加一定量的Al13和AlCl3。选择最佳药剂投加量和相应原水中有机物浓度,反应开始后,首先通过10 min慢速搅拌,令HA絮体生长。随后加入NaF溶液,令体系中氟化物质量浓度达到8 mg·L−1。此时,氟离子的吸附作用和三元络合共沉淀作用开始发生。慢速搅拌10 min,让反应充分进行。在随后的30 min内,静置水样令絮体充分沉降。自加入NaF起,在实验进行的40 min以内,以一定的时间间隔自烧杯中取液面下2 cm处水样,经0.45 μm滤膜迅速过滤后,采用离子选择电极对氟离子浓度进行实时监控。
4)样品分析与测试。沉后水中的氟化物浓度通过离子选择电极进行测试,余铝则通过电感耦合等离子体发射光谱法确定。本研究采用ESI-TOF-MS对混凝剂和沉后水中铝形态以及铝氟络合物进行分析。所有ESI-TOF-MS数据均在正离子模式下扫描。具体测试条件为:毛细管电压3.5 kV,样品锥电压90 V(40、70和150 V),抽锥电压5 V,源温度120 ℃,脱溶剂温度150 ℃,氮气流速300 L·h−1,进样流速10 μL·min−1,质谱扫描范围50~1 000,使用甲酸钠对谱图进行位移校正,在测试过程中利用亮氨酸-脑啡肽溶液进行实时校准[25]。
在中性(pH=7.0)和碱性(pH=8.0)的初始酸碱度条件下,经过混凝程序和沉降后,混凝剂水解产物(无氟)和絮体(有氟)聚集在烧杯底部。经离心后,将产物冷冻干燥,用于后续分析。利用傅立叶变换红外光谱仪和X射线光电子能谱仪对水解产物和絮体化学成分进行分析。
-
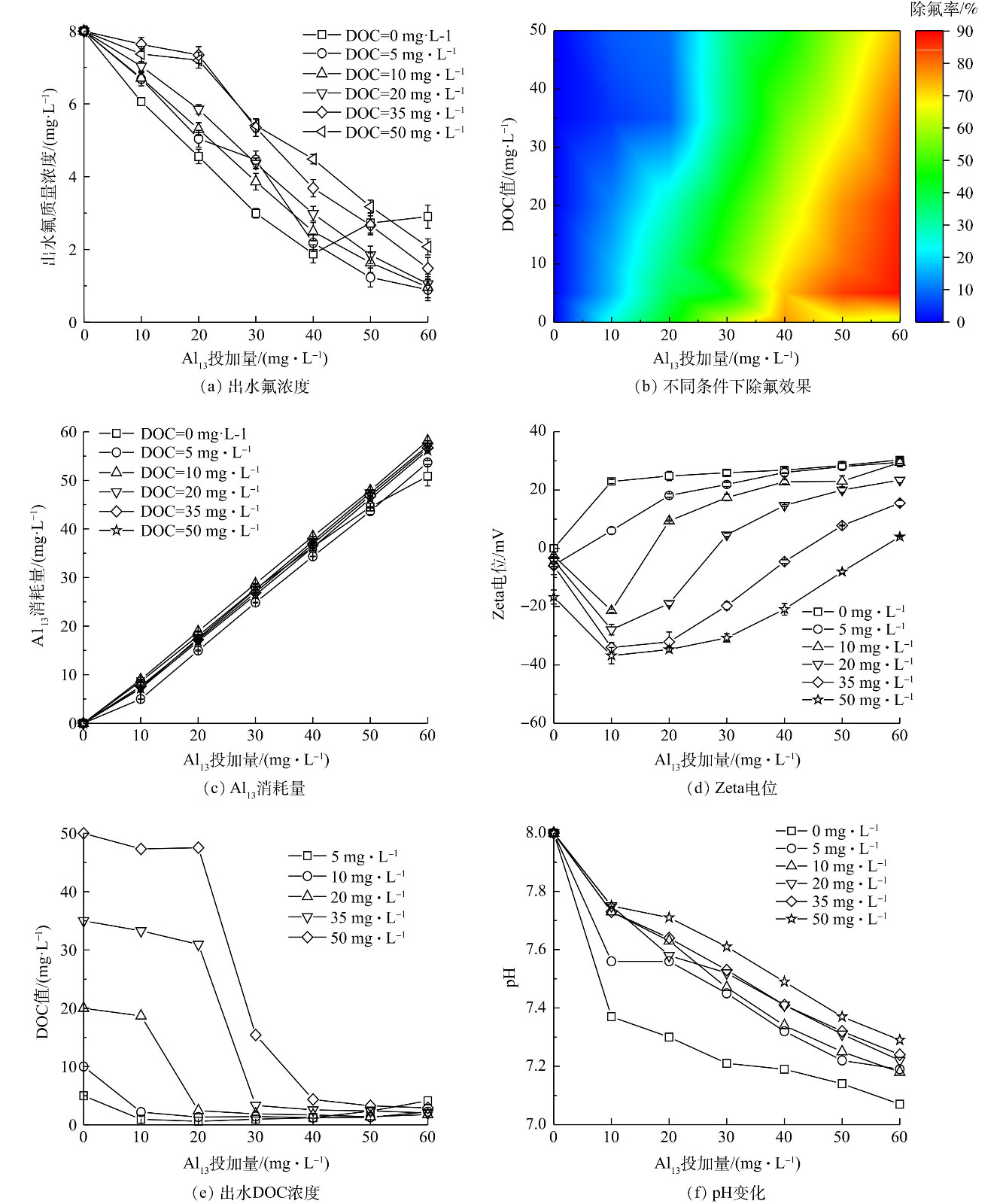
1) Al13混凝效果的影响分析。图1反映了HA对Al13混凝的影响作用。由图1(a)可知,投加Al13所得沉后水中,氟离子浓度随Al13投加量的增加先降后升,并在投加量为40 mg·L−1时达到最优除氟效果。向水体中加入HA,出水氟离子浓度随投加量增加持续降低。由图1(b)可见,在高投加量的混凝剂下,HA对Al13混凝除氟有促进作用,最优除氟率在90%以上,而在投加量低时表现出的则是抑制作用。图1(c)反映了混凝过程中Al13的消耗量的变化情况。各体系下混凝剂消耗量基本等于投加量,可以得出出水中的余铝浓度均较低,尤其是在含HA的体系中,在各投加量下余铝浓度始终接近斜率为1的直线。值得注意的是,Al13在处理纯氟水后残留余铝浓度较高,这是因为HA的引入会消耗掉过量的混凝剂,从而降低余铝残留。
图1(d)为各投加量下的Zeta电位。未投加混凝剂的纯氟水电位接近于0,在Al13混凝作用发生后,电位随投加量增加先升高后趋稳。这是因为HA携带负电荷,因此,体系的Zeta电位降低。在较高HA浓度的含氟废水中,加入Al13后Zeta电位首先出现下降趋势,这是由于体系中原本分散的HA在Al13的电中和作用下逐渐聚集成微絮体,令仪器产生较强的信号响应,随着投加量的增加,Zeta电位在达到最低点后,呈现出稳定上升的态势。图1(f)所示为各体系下的出水pH随投加量变化曲线。纯氟水中投加60 mg·L−1 Al13混凝处理后,pH从8.0降低到7.0左右。总体而言,氟离子和有机物混合废水中HA浓度越高,出水pH下降幅度越小,这是由于大部分混凝剂与污染物发生反应,只有少量发生水解反应产生氢离子。
2) AlCl3混凝效果的影响分析。图2反映了在不同HA浓度的含氟废水中AlCl3的混凝表现。如图2(a)和图2(b)所示,随着AlCl3投加量的增加,除氟率表现出先增后降的趋势。HA对除氟率的优化作用较小,仅当投加量超过30 mg·L−1时表现出一定的促进作用。AlCl3药剂消耗情况如图2(c)所示。在纯氟水中,AlCl3消耗量始终低于5 mg·L−1,说明出水余铝浓度较高。而在有HA共存的混合废水中,AlCl3的消耗量相对更高,且HA浓度越高消耗量越大。然而在高投加量下,AlCl3消耗量呈现下降趋势。究其原因,可能是高投量下水体pH的改变干扰了各铝形态与污染物的结合,导致药剂以余铝的形式继续存在于出水中。
图2(d)为AlCl3混凝过程中Zeta电位随药剂投加量的变化情况。随AlCl3投加量的增加,混合废水中Zeta电位先降后升,与Al13体系下的趋势较为接近,而纯氟水中Zeta电位则先上升后微弱下降。AlCl3作用下各模拟水中的有机物去除情况如图2(e)所示。与氟化物去除率相似,体系的DOC值也经历了先下降后上升的趋势。由图2(f)可知,AlCl3对水体的酸化效应较Al13更为明显。结合图2(a)和图2(b)的变化趋势,发现在AlCl3投加量超过30 mg·L−1后,pH低于6.0,会对抑制混凝过程,氟化物和HA的去除率均有所下降。这是因为AlCl3的混凝主要是由表面吸附和卷扫完成的,其工作最佳pH为6.0~7.0,此时铝溶解度最小,相应的有最大量无定形的Al(OH)3卷扫絮凝,因此,在该pH范围内混凝剂的去除效率较高[26-27]。
3) AlCl3和Al13混凝效果比较分析。通过AlCl3和Al13在不同HA浓度的含氟废水中的混凝表现,可以总结得出共性规律:纯氟水中的除氟率随投加量增加,先升高后下降;较高的混凝剂投加量下,共存的HA组分促进混凝除氟,而在低药剂投加量下则抑制了除氟作用;另外,由于HA的存在,在低投加量下产生的絮体,会导致体系Zeta电位的下降,随着投加量的进一步升高,电位呈现稳定上升趋势。然而,由于两种药剂的聚合度、分子量和水解特性存在较大区别,二者在不同HA浓度的含氟废水中的混凝表现也有较大差异。相比于AlCl3而言,Al13混凝伴随着较低的余铝浓度,也即预聚合的Al13更大程度上参与了与污染物的反应,随污染物一同聚集、沉降进入絮体中[28];另外,投加AlCl3对pH影响较大,如投加60 mg·L−1的AlCl3,会使pH从8.0下降到4.7左右,而投加Al13时,pH能稳定维持在中、碱性条件;Al13作用下的DOC值随投加量的增加呈现出相对稳定的降低趋势,而在AlCl3作用下,该趋势会在高投加量下发生明显上升;最后,在一定腐殖酸浓度和混凝剂投加量下,AlCl3和Al13二者均存在着最优促进除氟的作用区间,也即在特定条件下,混凝剂在复合污染水中达到了优于纯氟水中的混凝除氟表现。对于AlCl3而言,这一最优促进除氟作用区间存在于高DOC值下,即较高的HA对除氟效果的促进效应相对明显;而对于Al13而言,该作用区间存在于低DOC值下,也即HA浓度越低则越能促进氟离子的混凝去除。
-
为了探究除氟率、DOC去除率和其他水质参数之间的关系,采用相关分析对其投加混凝剂后的水质指标进行综合解析。由表2可知,在无HA时,氟化物去除率与Al13投加量和消耗量都呈现出显著正相关关系,说明在实验涉及的Al13投加量范围下,药剂能够充分地在水体中发挥除氟作用。此外,氟化物去除率与体系Zeta电位也呈现显著的正相关关系,且该相关性在0.1水平显著,这证实了带正电的水解产物或絮体对溶解态氟离子实现了去除。当在水体系中引入低质量浓度的HA(5 mg·L−1 DOC),HA的去除率与混凝剂消耗量并无显著相关关系,说明体系中的HA未对投加的混凝剂产生额外消耗。另外结合图1(e)可知,这一浓度的HA在较低投加量下就能够得以稳定去除,而在此水质状况下,氟化物去除率仍然维持在较高水平,且氟化物去除率仍与混凝剂消耗量呈显著正相关(在0.05水平显著),这表示低浓度HA并没有对氟化物的混凝去除产生较大干扰。并且当模拟水中的DOC值增加到10 mg·L−1,氟化物去除率仍然与混凝剂消耗量和Zeta电位呈现显著正相关关系。较高的相关性进一步证实了低浓度的HA和氟的竞争作用相对较小。
当原水中DOC值增加到20 mg·L−1,DOC去除率开始与混凝消耗量呈现正相关,且这一相关性在0.1水平显著,这说明体系中的HA对氟的去除产生了竞争效应。值得注意的是,此时氟化物和HA去除率在0.1水平上同样存在着正相关关系。由此可以推测得出,除竞争关系之外,两种污染物之间还存在着协同去除的关系,这一协同关系的内在机制可能是Al13-HA-F的三元络合作用。当DOC值增加到35 mg·L−1,HA和氟化物去除率的正相关关系显著性进一步增加,正相关关系在0.05水平上显著。另外,两种污染物的去除率均与混凝剂消耗量呈现显著正相关。进一步增加DOC值到50 mg·L−1,HA去除率与混凝剂投加量、消耗量以及氟化物去除率都继续保持显著正相关关系。
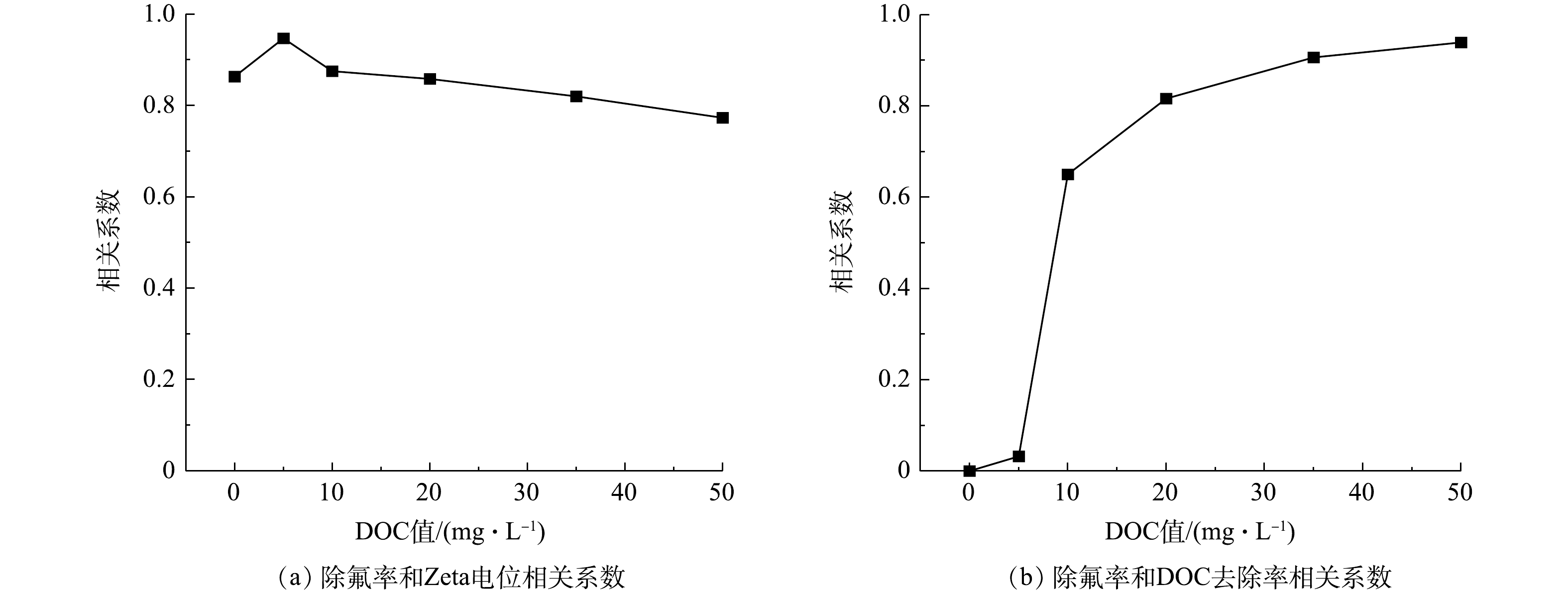
如图3所示,随共存有机物浓度上升,氟化物去除率与Zeta电位始终存在较为显著的相关关系。这一相关关系进一步证实,Al13主要依靠预制分子与氟化物的共沉淀作用,与氟离子生成固相态微絮体(铝氟共沉淀),再经过静沉离开水相。然而,随着体系中HA浓度的增加,这一相关系数呈现下降趋势,这意味着体系中存在其他作用机制促进了氟化物的去除。另外,由于HA分子携带负电荷,其与铝氟共沉淀结合之后,会降低絮体表面的正电荷密度,进而对Zeta电位以及相关系数产生影响。值得注意的是,对比纯氟水与DOC值为5 mg·L−1的混合废水,Zeta电位与氟化物去除率的相关系数从0.863升高到0.947,这说明HA的引入有促进成核和絮体形成的作用。图3(b)反映了不同模拟水中的氟化物去除率与DOC去除率相关系数的变化情况。DOC值在0~50 mg·L−1内,2种污染物的去除率相关系数随着有机物浓度的升高而升高,说明2种污染物的去除逐渐趋于同步,三元络合作用逐渐占据主导作用。
-
图4(a)以Al13在纯氟水中的混凝除氟表现为基准(赋值为0),反映了DOC值对Al13除氟率的影响。可以发现,混凝剂在高投加量时,HA对除氟有促进作用,但在低投加量下存在抑制作用。也就是说,该体系中存在着三元络合作用,从而促进氟化物被Al13混凝去除。同时,HA的存在使得混凝剂被额外消耗,进而对氟化物的混凝去除产生干扰。图4(b)所示则为不同HA浓度的含氟废水在不同Al13投加量下出水紫外吸光度(SUVA)情况。当DOC值越低,Al13投加量越大时则SUVA较低。对比图4(a)与图4(b)后,可以发现在三元络合作用最为显著的反应条件下沉后水的SUVA值最低,水体中的疏水性组分最大程度地参与了混凝沉淀反应,促进了氟化物的去除。因而可以初步判断,参与三元络合的最关键组分就是有机物中的疏水性组分。
-
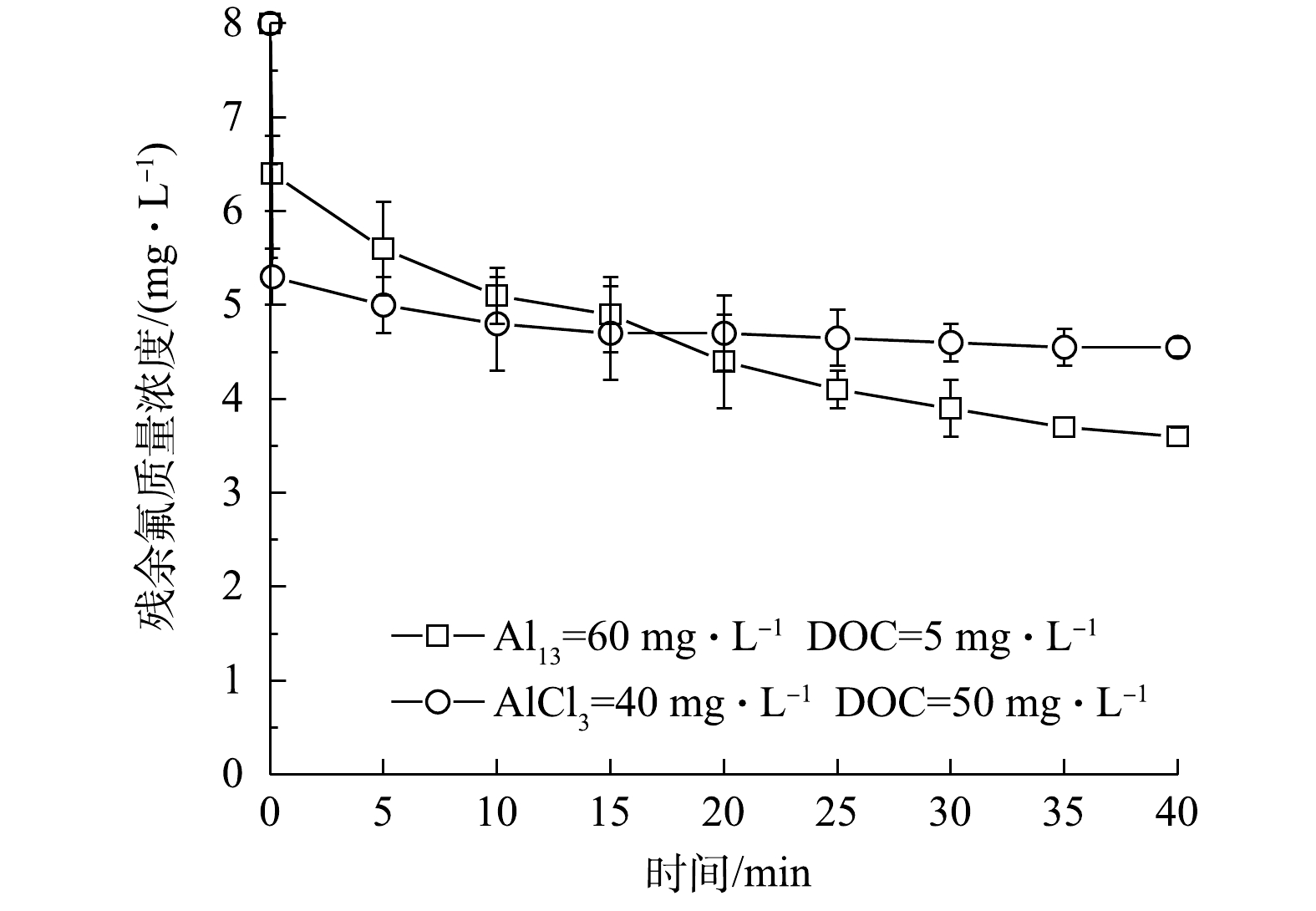
图5反映了2种混凝剂作用时上清液中氟离子浓度随反应时间的变化情况。AlCl3在无氟离子时就可以与HA形成肉眼可见且稳定的絮体,而在引入氟离子后,能对其进行有效吸附,氟离子浓度迅速达到相对平衡;然而,Al13在无氟离子时无法与HA形成明显的絮体,直到向体系中引入NaF后,才引发反应产生絮体。这一现象证实了Al13作用体系下三元络合作用的存在。另外,不同于AlCl3絮体的快速吸附作用, Al13作用下的氟离子浓度下降速度缓慢,且直到40 min结束时仍然呈现出浓度下降趋势,从而进一步支持了三元络合和沉淀作用的存在。虽然Al13组的氟化物浓度下降速度较慢,但从去除效果来看,水中的氟离子浓度水平在经过10 min的慢速搅拌和30 min的静沉后相对AlCl3组更低,即Al13在三元络合沉淀作用主导下体现出更优的除氟效果。
-
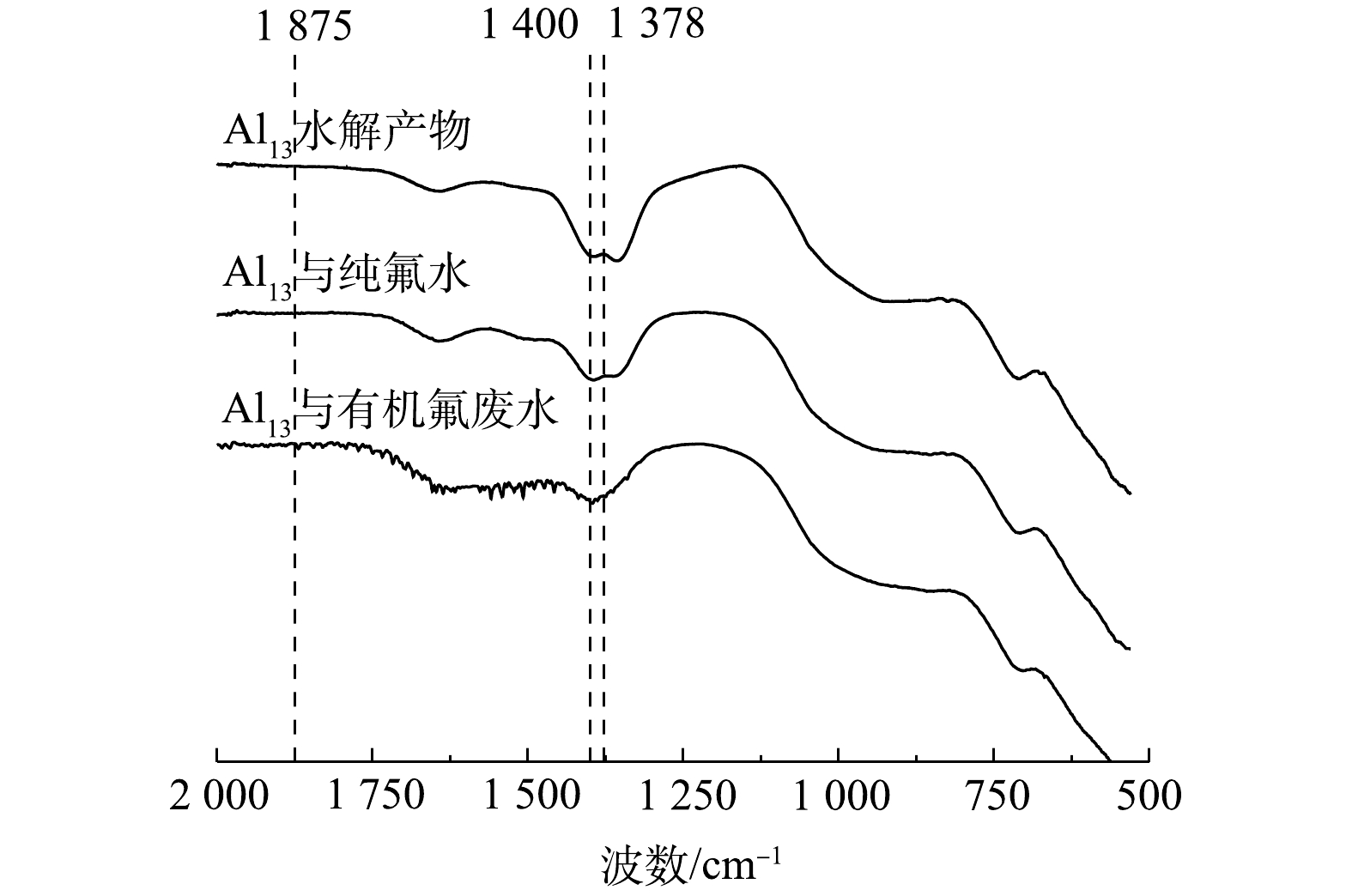
图6为在pH=8.0条件下的Al13水解产物及其在纯氟水与混合废水中絮体的红外光谱图。药剂投加量固定为50 mg·L−1,混合废水中初始DOC值为5 mg·L−1。从谱图对比中可以明显看出,相较于水解产物和含氟絮体,混合废水中对应絮体的红外谱峰更为复杂,在1 400~1 875 cm−1内分布有若干个尖峰。HA作为由长链脂族结构链接多个芳香环而形成的大分子物质,其分子结构中存在大量羧基和羟基活性官能团[29-30]。因此,当其参与絮体的形成,必然在红外谱图中显示出复杂而丰富的吸收峰响应。已有研究表明,氟离子的存在会促进Al13与HA中酚羟基的络合,从而促进HA被Al13混凝去除,这也解释了特定条件下氟离子引发三元络合及絮体产生的这一实验现象[31-32]。在1 378 cm−1处,在体系中引入HA之后,该位置原有的峰与代表酚羟基弯曲振动的峰发生重合,使原本较为平缓的吸收峰变得更为尖锐[33-34]。另外,由于该官能团参与了与金属混凝剂的络合,其化学位置的改变也致使代表酚羟基的这一峰向低波数处进行位移。
-
1)在纯氟水中,随着AlCl3和Al13投加量增加,除氟率均先升后降。而在不同HA浓度的含氟废水中,AlCl3和Al13均存在促进除氟的作用区间,但Al13比AlCl3有更好的除氟效果(在混凝剂质量浓度超过35 mg·L−1后)、更低的余铝浓度和更轻微的pH影响。当DOC为5 mg·L−1、Al13的质量浓度为60 mg·L−1时,其最优除氟率可达88.8%。而在DOC值为35 mg·L−1,混凝剂投加质量浓度为35 mg·L−1时,AlCl3的除氟率最优,为75%。
2)有机物存在下,Al13的除氟内在机制为Al13-HA-F的三元络合作用,HA的引入可促进成核和絮体形成。而SUVA值与动态吸附实验分析结果表明,参与Al-HA-F三元络合产物形成的主要是有机物中疏水性组分。
3)除氟效果是有机物的竞争作用与三元络合促进作用的动态平衡结果,并且氟离子的存在能够促进HA中酚羟基与Al13的络合,促进HA被Al13混凝去除。
AlCl3与Al13对不同HA浓度含氟废水的处理效果及作用机理
Treatment effect and mechanism of treatment of fluorine-containing wastewater with different HA concentrations by AlCl3 and Al13
-
摘要: 混凝是一种重要的除氟工艺。以不同腐殖质(HA)浓度的含氟废水为研究对象,对比高正电性分子[AlO4Al12(OH)24(H2O)12]7+(Al13)和氯化铝(AlCl3)2种不同形态混凝剂的除氟效果。结果表明,在纯氟水中,增加混凝剂投加量,除氟率均为先升高后下降,而在HA存在的条件时,混凝剂的除氟效果是氟和有机物的竞争作用与三元络合促进作用在动态平衡下的结果。氟离子和有机物混合废水中的氟离子能够促使有机质中酚羟基与Al13的络合。Al13在混凝过程中会形成三元络合物,促使氟离子脱离水相,因此,除氟效果更好。当溶解性有机碳质量浓度为5 mg·L−1时,Al13的最高除氟率可达88.8%。Abstract: Coagulation is an important fluoride removal process. In this study, fluorine-containing wastewater with different HA concentrations was taken as the research object, and the fluoride removal effects by high positive molecular [AlO4Al12(OH)24(H2O)12]7+ (Al13) and aluminum chloride (AlCl3) were compared. The results showed that in pure fluorine water, with the increase of coagulant dosage, the fluoride removal rate increased at first and then decreased; while in the presence of HA, the fluoride removal effect of coagulants was the result of the competition between fluorine and organic matter and the promotion of ternary complexation under dynamic equilibrium. The fluoride ion in the mixed wastewater of fluoride ion and organic matter could promote the complexation of phenolic hydroxyl groups in organic matter with Al13. In the process of coagulation, Al13 could form a ternary complex to promote the separation of fluoride from the aqueous phase, so it had a better fluoride removal effect. When the mass concentration of dissolved organic carbon was 5 mg·L-1, the highest fluoride removal rate of Al13 reached 88.8%.
-
Key words:
- AlCl3 /
- Al13 /
- mixed wastewater /
- coagulation /
- defluorination
-
氟是一种人体必须的微量元素,适量的氟有利于预防骨质疏松和龋齿,但过量的摄入会导致骨髓畸形和脑损伤等疾病。在自然界中氟元素会在风化作用下进入地下水,人类活动如煤矿、玻璃陶瓷、半导体制造和电镀等工业,也会在生产制造过程中排出大量高浓度的含氟废水,之后与人类活动产生的有机污染物混合后,会形成较难处理的氟离子和有机物混合废水。针对此类废水,常用的处理方法有混凝法[1-2]、吸附法[3-4]、离子交换法[5-6]、电渗析法、反渗透膜法[7]和电絮凝法[8]等。吸附法具有反应速率快和工艺简单的优势,但仅在污染物浓度较低时有效,而且吸附剂的处理和再生成本较高[9]。离子交换法可以针对性地去除水中的氟离子,但无法去除有机物,且存在树脂再生困难和出现2次污染问题[10];膜处理法可以有效去除水中的氟离子和有机物,但其由于对设备要求高、滤膜易堵塞以及2次浓水处理困难等问题,在实际应用中会受到限制[11]。
以氯化铝(AlCl3)为代表的传统铝系混凝剂凭借着处理效果稳定和价格低廉的优点[12],广泛应用于中小型水处理措施中。但其存在絮体体积小且结构疏松、低温下混凝效果差、药剂投入量大和余铝不可控等问题,极少应用在大规模工程中[13]。高聚十三铝(Al13)是一种纳米级尺寸和高正电荷密度的溶胶大分子新型混凝剂,有极佳的混凝水解形态[14-16]。相比于传统的铝盐混凝剂,Al13经过预水解生成的产物更倾向于保持其化学特性,因此有较高的水解稳定性,并且其高正电荷密度的特点,更有利于接近和吸附污染物。此外,氢键和离子交换机制也进一步保证了高效的混凝效果。腐殖酸(HA)是自然界中最常见的有机质[17-18],由动植物遗骸经过微生物的分解和转化形成,其分子质量分布在800~6 000 Da,通常作为模型物质替代天然有机物开展研究。研究表明,AlCl3和Al13作为铝系混凝剂对水中的氟离子具有高效的混凝去除能力[19]。并且借助电中和、网捕卷扫等机理铝系混凝剂也具有一定的去除有机物的能力[20]。然而,不同铝系混凝剂在处理氟离子和有机物混合废水时,均会面临混凝剂性能下降,氟离子去除效率明显下降的问题。
为揭示不同铝系混凝剂在处理氟离子和有机物混合废水时的作用机理,解释混凝剂在处理氟离子和有机物混合废水时,氟离子去除效率下降的现象,本研究制备不同HA浓度的含氟废水作为实验水体进行实验[21-22],通过对比不同形态铝混凝剂对混合废水中氟离子的去除效果,探讨其内在的作用机理,以期对氟离子与有机物混合废水处理或供水厂工艺选择提供参考。
1. 材料与方法
1.1 试剂和仪器
六水合氯化铝(AlCl3·6H2O)、氢氧化钠(NaOH)、盐酸(HCl)、氟化钠(NaF)、硝酸钠(NaNO3)、二水合氯化钡(BaCl2·2H2O)、硫酸钠(Na2SO4)和乙酸钠(CH3COONa)分析纯,均购自国药集团化学药剂有限公司。二水合柠檬酸三钠(Na3C6H5O7·2H2O)分析纯、HA ≥ 90%购自阿拉丁试剂公司,Ferron试剂分析纯,购自Sigma-Aldrich试剂公司。主要仪器有电喷雾四极杆飞行时间质谱(ESI-TOF-MS)(ACQUITY UPLC/Xevo G2 Q TOF, Waters, USA)、紫外可见分光光度计(UH5300, ShimadZu, Japan)、六联搅拌仪(MY 3000-2J/4J,梅宇,中国)、Zeta电位分析仪(ZetasiZer Nano ZS90, Malvern, UK)、离子选择电极(PHS-3C,雷磁,中国)、电感耦合等离子体发射光谱法(ICP-OES)(ICPE-9800, ShimadZu, Japan)、利用傅立叶变换红外光谱仪(FTIR)(Nicolet 8700, ThermoFisher, USA)、X射线光电子能谱仪(XPS)(ESCALAB250Xi,ThermoFisher, USA)、总有机碳分析仪(ShimadZu,TOC-L,日本)。
1.2 混凝剂的选择与表征
实验中AlCl3溶液由药品直接配制。Al13由碱化度2.2的聚合氯化铝溶液,利用硫酸钠和氯化钡沉淀置换法提纯得到[23],在确定产物纯度后,利用超纯水稀释质量浓度至2.5 g·L−1。2种混凝剂均为现用现制,浓度以Al的质量计算。将Al13和AlCl3溶液稀释至16.2 mg·L−1后,利用Al-Ferron逐时络合比色法[24]并结合紫外可见分光光度计测定2种混凝剂中Al的形态(表1)。结果表明,AlCl3中主要的铝形态为Ala,而Al13中主要铝形态为Alb。
表 1 混凝剂的Al形态分布Table 1. Al species of the coagulants used in this studyAl形态 Ala/% Alb/% Alc/% AlCl3 95.59 3.87 0.54 Al13 3.11 94.32 2.57 1.3 实验方法
1)模拟水样配置。取10.0 g HA粉末分散于1 L超纯水中,加入0.4 g NaOH不断搅拌至HA溶解。持续搅拌5 h后,用0.45 μm滤膜过滤。利用总有机碳分析仪确定储备液的溶解性有机碳(DOC) 质量浓度,为2 791.7 mg·L−1。定量加入去离子水,将储备液稀释至DOC为2 000.0 mg·L−1,即可得HA储备液。将NaF药品置于110 ℃的烘箱中干燥4 h后,精确称量并配置成含氟离子浓度为1.0 g·L−1的NaF储备液。在超纯水中引入NaF储备液,将氟离子质量浓度确定为8.0 mg·L−1。再加入不同浓度HA储备液,制成6种模拟水样,DOC值分别为0、5、10、20、35和50 mg·L−1。上述6种水样中,均需加入10.0 mmol·L−1 NaNO3和4.0 mmol·L−1 NaHCO3溶液以调节离子强度和碱度。最终,利用0.1 mol·L−1的HCl和NaOH溶液调节水样pH至8.0。
2)混凝实验。室温下使用搅拌仪进行混凝实验。在添加混凝剂之前,首先将500 mL模拟水样以250 rpm的速度搅拌30 s。投加药剂后,以200 r·min−1的转速搅拌90 s充分混合。测试样品Zeta电位3次并取平均值。降低搅拌速度至40 r·min−1,慢搅10 min令絮体生长。沉降30 min后,利用0.45 μm滤膜对上清液进行过滤,对渗出液中氟化物质量浓度、DOC值和余铝质量浓度进行测定。
3)动态吸附实验。通过动态吸附实验探究氟离子、有机物和Al13三者之间的作用顺序。在无氟HA溶液中,投加一定量的Al13和AlCl3。选择最佳药剂投加量和相应原水中有机物浓度,反应开始后,首先通过10 min慢速搅拌,令HA絮体生长。随后加入NaF溶液,令体系中氟化物质量浓度达到8 mg·L−1。此时,氟离子的吸附作用和三元络合共沉淀作用开始发生。慢速搅拌10 min,让反应充分进行。在随后的30 min内,静置水样令絮体充分沉降。自加入NaF起,在实验进行的40 min以内,以一定的时间间隔自烧杯中取液面下2 cm处水样,经0.45 μm滤膜迅速过滤后,采用离子选择电极对氟离子浓度进行实时监控。
4)样品分析与测试。沉后水中的氟化物浓度通过离子选择电极进行测试,余铝则通过电感耦合等离子体发射光谱法确定。本研究采用ESI-TOF-MS对混凝剂和沉后水中铝形态以及铝氟络合物进行分析。所有ESI-TOF-MS数据均在正离子模式下扫描。具体测试条件为:毛细管电压3.5 kV,样品锥电压90 V(40、70和150 V),抽锥电压5 V,源温度120 ℃,脱溶剂温度150 ℃,氮气流速300 L·h−1,进样流速10 μL·min−1,质谱扫描范围50~1 000,使用甲酸钠对谱图进行位移校正,在测试过程中利用亮氨酸-脑啡肽溶液进行实时校准[25]。
在中性(pH=7.0)和碱性(pH=8.0)的初始酸碱度条件下,经过混凝程序和沉降后,混凝剂水解产物(无氟)和絮体(有氟)聚集在烧杯底部。经离心后,将产物冷冻干燥,用于后续分析。利用傅立叶变换红外光谱仪和X射线光电子能谱仪对水解产物和絮体化学成分进行分析。
2. 结果与讨论
2.1 铝系混凝剂混凝效果的影响分析
1) Al13混凝效果的影响分析。图1反映了HA对Al13混凝的影响作用。由图1(a)可知,投加Al13所得沉后水中,氟离子浓度随Al13投加量的增加先降后升,并在投加量为40 mg·L−1时达到最优除氟效果。向水体中加入HA,出水氟离子浓度随投加量增加持续降低。由图1(b)可见,在高投加量的混凝剂下,HA对Al13混凝除氟有促进作用,最优除氟率在90%以上,而在投加量低时表现出的则是抑制作用。图1(c)反映了混凝过程中Al13的消耗量的变化情况。各体系下混凝剂消耗量基本等于投加量,可以得出出水中的余铝浓度均较低,尤其是在含HA的体系中,在各投加量下余铝浓度始终接近斜率为1的直线。值得注意的是,Al13在处理纯氟水后残留余铝浓度较高,这是因为HA的引入会消耗掉过量的混凝剂,从而降低余铝残留。
图1(d)为各投加量下的Zeta电位。未投加混凝剂的纯氟水电位接近于0,在Al13混凝作用发生后,电位随投加量增加先升高后趋稳。这是因为HA携带负电荷,因此,体系的Zeta电位降低。在较高HA浓度的含氟废水中,加入Al13后Zeta电位首先出现下降趋势,这是由于体系中原本分散的HA在Al13的电中和作用下逐渐聚集成微絮体,令仪器产生较强的信号响应,随着投加量的增加,Zeta电位在达到最低点后,呈现出稳定上升的态势。图1(f)所示为各体系下的出水pH随投加量变化曲线。纯氟水中投加60 mg·L−1 Al13混凝处理后,pH从8.0降低到7.0左右。总体而言,氟离子和有机物混合废水中HA浓度越高,出水pH下降幅度越小,这是由于大部分混凝剂与污染物发生反应,只有少量发生水解反应产生氢离子。
2) AlCl3混凝效果的影响分析。图2反映了在不同HA浓度的含氟废水中AlCl3的混凝表现。如图2(a)和图2(b)所示,随着AlCl3投加量的增加,除氟率表现出先增后降的趋势。HA对除氟率的优化作用较小,仅当投加量超过30 mg·L−1时表现出一定的促进作用。AlCl3药剂消耗情况如图2(c)所示。在纯氟水中,AlCl3消耗量始终低于5 mg·L−1,说明出水余铝浓度较高。而在有HA共存的混合废水中,AlCl3的消耗量相对更高,且HA浓度越高消耗量越大。然而在高投加量下,AlCl3消耗量呈现下降趋势。究其原因,可能是高投量下水体pH的改变干扰了各铝形态与污染物的结合,导致药剂以余铝的形式继续存在于出水中。
图2(d)为AlCl3混凝过程中Zeta电位随药剂投加量的变化情况。随AlCl3投加量的增加,混合废水中Zeta电位先降后升,与Al13体系下的趋势较为接近,而纯氟水中Zeta电位则先上升后微弱下降。AlCl3作用下各模拟水中的有机物去除情况如图2(e)所示。与氟化物去除率相似,体系的DOC值也经历了先下降后上升的趋势。由图2(f)可知,AlCl3对水体的酸化效应较Al13更为明显。结合图2(a)和图2(b)的变化趋势,发现在AlCl3投加量超过30 mg·L−1后,pH低于6.0,会对抑制混凝过程,氟化物和HA的去除率均有所下降。这是因为AlCl3的混凝主要是由表面吸附和卷扫完成的,其工作最佳pH为6.0~7.0,此时铝溶解度最小,相应的有最大量无定形的Al(OH)3卷扫絮凝,因此,在该pH范围内混凝剂的去除效率较高[26-27]。
3) AlCl3和Al13混凝效果比较分析。通过AlCl3和Al13在不同HA浓度的含氟废水中的混凝表现,可以总结得出共性规律:纯氟水中的除氟率随投加量增加,先升高后下降;较高的混凝剂投加量下,共存的HA组分促进混凝除氟,而在低药剂投加量下则抑制了除氟作用;另外,由于HA的存在,在低投加量下产生的絮体,会导致体系Zeta电位的下降,随着投加量的进一步升高,电位呈现稳定上升趋势。然而,由于两种药剂的聚合度、分子量和水解特性存在较大区别,二者在不同HA浓度的含氟废水中的混凝表现也有较大差异。相比于AlCl3而言,Al13混凝伴随着较低的余铝浓度,也即预聚合的Al13更大程度上参与了与污染物的反应,随污染物一同聚集、沉降进入絮体中[28];另外,投加AlCl3对pH影响较大,如投加60 mg·L−1的AlCl3,会使pH从8.0下降到4.7左右,而投加Al13时,pH能稳定维持在中、碱性条件;Al13作用下的DOC值随投加量的增加呈现出相对稳定的降低趋势,而在AlCl3作用下,该趋势会在高投加量下发生明显上升;最后,在一定腐殖酸浓度和混凝剂投加量下,AlCl3和Al13二者均存在着最优促进除氟的作用区间,也即在特定条件下,混凝剂在复合污染水中达到了优于纯氟水中的混凝除氟表现。对于AlCl3而言,这一最优促进除氟作用区间存在于高DOC值下,即较高的HA对除氟效果的促进效应相对明显;而对于Al13而言,该作用区间存在于低DOC值下,也即HA浓度越低则越能促进氟离子的混凝去除。
2.2 Al13混凝除氟效果相关分析
为了探究除氟率、DOC去除率和其他水质参数之间的关系,采用相关分析对其投加混凝剂后的水质指标进行综合解析。由表2可知,在无HA时,氟化物去除率与Al13投加量和消耗量都呈现出显著正相关关系,说明在实验涉及的Al13投加量范围下,药剂能够充分地在水体中发挥除氟作用。此外,氟化物去除率与体系Zeta电位也呈现显著的正相关关系,且该相关性在0.1水平显著,这证实了带正电的水解产物或絮体对溶解态氟离子实现了去除。当在水体系中引入低质量浓度的HA(5 mg·L−1 DOC),HA的去除率与混凝剂消耗量并无显著相关关系,说明体系中的HA未对投加的混凝剂产生额外消耗。另外结合图1(e)可知,这一浓度的HA在较低投加量下就能够得以稳定去除,而在此水质状况下,氟化物去除率仍然维持在较高水平,且氟化物去除率仍与混凝剂消耗量呈显著正相关(在0.05水平显著),这表示低浓度HA并没有对氟化物的混凝去除产生较大干扰。并且当模拟水中的DOC值增加到10 mg·L−1,氟化物去除率仍然与混凝剂消耗量和Zeta电位呈现显著正相关关系。较高的相关性进一步证实了低浓度的HA和氟的竞争作用相对较小。
表 2 不同DOC值下除氟率与DOC去除率与各水质参数相关分析Table 2. Correlation analysis of fluoride removal rate and DOC removal rate with water quality parameters at different DOC valuesDOC /(mg·L−1) 种类 Al13投加量 Zeta电位 余铝 铝消耗量 0 除氟率 0.869* 0.863* 0.674 0.891** DOC去除率 — — — — 5 除氟率 0.987** 0.947** 0.229 0.966** DOC去除率 -0.038 0.297 0.842 -0.147 10 除氟率 0.993** 0.875** 0.933** 0.993** DOC去除率 0.607 0.384 0.819* 0.598 20 除氟率 0.997** 0.858* 0.624 0.998** DOC去除率 0.811* 0.623 0.639 0.805* 35 除氟率 0.979** 0.820* 0.666 0.984** DOC去除率 0.892** 0.654 0.709 0.891** 50 除氟率 0.985** 0.773* 0.701 0.989** DOC去除率 0.924** 0.635 0.709 0.925** 注:*相关性在0.05水平显著,**相关性在0.1水平显著。 当原水中DOC值增加到20 mg·L−1,DOC去除率开始与混凝消耗量呈现正相关,且这一相关性在0.1水平显著,这说明体系中的HA对氟的去除产生了竞争效应。值得注意的是,此时氟化物和HA去除率在0.1水平上同样存在着正相关关系。由此可以推测得出,除竞争关系之外,两种污染物之间还存在着协同去除的关系,这一协同关系的内在机制可能是Al13-HA-F的三元络合作用。当DOC值增加到35 mg·L−1,HA和氟化物去除率的正相关关系显著性进一步增加,正相关关系在0.05水平上显著。另外,两种污染物的去除率均与混凝剂消耗量呈现显著正相关。进一步增加DOC值到50 mg·L−1,HA去除率与混凝剂投加量、消耗量以及氟化物去除率都继续保持显著正相关关系。
如图3所示,随共存有机物浓度上升,氟化物去除率与Zeta电位始终存在较为显著的相关关系。这一相关关系进一步证实,Al13主要依靠预制分子与氟化物的共沉淀作用,与氟离子生成固相态微絮体(铝氟共沉淀),再经过静沉离开水相。然而,随着体系中HA浓度的增加,这一相关系数呈现下降趋势,这意味着体系中存在其他作用机制促进了氟化物的去除。另外,由于HA分子携带负电荷,其与铝氟共沉淀结合之后,会降低絮体表面的正电荷密度,进而对Zeta电位以及相关系数产生影响。值得注意的是,对比纯氟水与DOC值为5 mg·L−1的混合废水,Zeta电位与氟化物去除率的相关系数从0.863升高到0.947,这说明HA的引入有促进成核和絮体形成的作用。图3(b)反映了不同模拟水中的氟化物去除率与DOC去除率相关系数的变化情况。DOC值在0~50 mg·L−1内,2种污染物的去除率相关系数随着有机物浓度的升高而升高,说明2种污染物的去除逐渐趋于同步,三元络合作用逐渐占据主导作用。
2.3 SUVA值与三元络合作用解析
图4(a)以Al13在纯氟水中的混凝除氟表现为基准(赋值为0),反映了DOC值对Al13除氟率的影响。可以发现,混凝剂在高投加量时,HA对除氟有促进作用,但在低投加量下存在抑制作用。也就是说,该体系中存在着三元络合作用,从而促进氟化物被Al13混凝去除。同时,HA的存在使得混凝剂被额外消耗,进而对氟化物的混凝去除产生干扰。图4(b)所示则为不同HA浓度的含氟废水在不同Al13投加量下出水紫外吸光度(SUVA)情况。当DOC值越低,Al13投加量越大时则SUVA较低。对比图4(a)与图4(b)后,可以发现在三元络合作用最为显著的反应条件下沉后水的SUVA值最低,水体中的疏水性组分最大程度地参与了混凝沉淀反应,促进了氟化物的去除。因而可以初步判断,参与三元络合的最关键组分就是有机物中的疏水性组分。
2.4 Al-HA絮体对氟离子吸附作用解析
图5反映了2种混凝剂作用时上清液中氟离子浓度随反应时间的变化情况。AlCl3在无氟离子时就可以与HA形成肉眼可见且稳定的絮体,而在引入氟离子后,能对其进行有效吸附,氟离子浓度迅速达到相对平衡;然而,Al13在无氟离子时无法与HA形成明显的絮体,直到向体系中引入NaF后,才引发反应产生絮体。这一现象证实了Al13作用体系下三元络合作用的存在。另外,不同于AlCl3絮体的快速吸附作用, Al13作用下的氟离子浓度下降速度缓慢,且直到40 min结束时仍然呈现出浓度下降趋势,从而进一步支持了三元络合和沉淀作用的存在。虽然Al13组的氟化物浓度下降速度较慢,但从去除效果来看,水中的氟离子浓度水平在经过10 min的慢速搅拌和30 min的静沉后相对AlCl3组更低,即Al13在三元络合沉淀作用主导下体现出更优的除氟效果。
2.5 絮体污染物红外光谱分析
图6为在pH=8.0条件下的Al13水解产物及其在纯氟水与混合废水中絮体的红外光谱图。药剂投加量固定为50 mg·L−1,混合废水中初始DOC值为5 mg·L−1。从谱图对比中可以明显看出,相较于水解产物和含氟絮体,混合废水中对应絮体的红外谱峰更为复杂,在1 400~1 875 cm−1内分布有若干个尖峰。HA作为由长链脂族结构链接多个芳香环而形成的大分子物质,其分子结构中存在大量羧基和羟基活性官能团[29-30]。因此,当其参与絮体的形成,必然在红外谱图中显示出复杂而丰富的吸收峰响应。已有研究表明,氟离子的存在会促进Al13与HA中酚羟基的络合,从而促进HA被Al13混凝去除,这也解释了特定条件下氟离子引发三元络合及絮体产生的这一实验现象[31-32]。在1 378 cm−1处,在体系中引入HA之后,该位置原有的峰与代表酚羟基弯曲振动的峰发生重合,使原本较为平缓的吸收峰变得更为尖锐[33-34]。另外,由于该官能团参与了与金属混凝剂的络合,其化学位置的改变也致使代表酚羟基的这一峰向低波数处进行位移。
3. 结论
1)在纯氟水中,随着AlCl3和Al13投加量增加,除氟率均先升后降。而在不同HA浓度的含氟废水中,AlCl3和Al13均存在促进除氟的作用区间,但Al13比AlCl3有更好的除氟效果(在混凝剂质量浓度超过35 mg·L−1后)、更低的余铝浓度和更轻微的pH影响。当DOC为5 mg·L−1、Al13的质量浓度为60 mg·L−1时,其最优除氟率可达88.8%。而在DOC值为35 mg·L−1,混凝剂投加质量浓度为35 mg·L−1时,AlCl3的除氟率最优,为75%。
2)有机物存在下,Al13的除氟内在机制为Al13-HA-F的三元络合作用,HA的引入可促进成核和絮体形成。而SUVA值与动态吸附实验分析结果表明,参与Al-HA-F三元络合产物形成的主要是有机物中疏水性组分。
3)除氟效果是有机物的竞争作用与三元络合促进作用的动态平衡结果,并且氟离子的存在能够促进HA中酚羟基与Al13的络合,促进HA被Al13混凝去除。
-
表 1 混凝剂的Al形态分布
Table 1. Al species of the coagulants used in this study
Al形态 Ala/% Alb/% Alc/% AlCl3 95.59 3.87 0.54 Al13 3.11 94.32 2.57 表 2 不同DOC值下除氟率与DOC去除率与各水质参数相关分析
Table 2. Correlation analysis of fluoride removal rate and DOC removal rate with water quality parameters at different DOC values
DOC /(mg·L−1) 种类 Al13投加量 Zeta电位 余铝 铝消耗量 0 除氟率 0.869* 0.863* 0.674 0.891** DOC去除率 — — — — 5 除氟率 0.987** 0.947** 0.229 0.966** DOC去除率 -0.038 0.297 0.842 -0.147 10 除氟率 0.993** 0.875** 0.933** 0.993** DOC去除率 0.607 0.384 0.819* 0.598 20 除氟率 0.997** 0.858* 0.624 0.998** DOC去除率 0.811* 0.623 0.639 0.805* 35 除氟率 0.979** 0.820* 0.666 0.984** DOC去除率 0.892** 0.654 0.709 0.891** 50 除氟率 0.985** 0.773* 0.701 0.989** DOC去除率 0.924** 0.635 0.709 0.925** 注:*相关性在0.05水平显著,**相关性在0.1水平显著。 -
[1] ABTAHI M, KOOLIVAND A, DOBARADARAN S, et al. Defluoridation of synthetic and natural waters by polyaluminum chloride-chitosan (PACl-ch) composite coagulant[J]. Water Science and Technology:Water Supply, 2018, 18(1): 259-269. doi: 10.2166/ws.2017.085 [2] CHO D W, HAN Y S, LEE J, et al. Water defluorination using granular composite synthesiZed via hydrothermal treatment of polyaluminum chloride (PAC) sludge[J]. Chemosphere, 2020, 247: 125899. doi: 10.1016/j.chemosphere.2020.125899 [3] ISLAM M, PATEL R K. Evaluation of removal efficiency of fluoride from aqueous solution using quick lime[J]. Journal of Hazardous Materials, 2007, 143(1/2): 303-310. doi: 10.1016/j.jhazmat.2006.09.030 [4] EL-SAID G F, EL-SADAAWY M M, ALY-ELDEEN M A. Adsorption isotherms and kinetic studies for the defluoridation from aqueous solution using eco-friendly raw marine green algae, Ulva lactuca[J]. Environmental Monitoring and Assessment, 2017, 190(1): 14. [5] ALKAN E, KR E, OKSUZ L. Plasma modification of the anion-exchange membrane and its influence on fluoride removal from water[J]. Separation & Purification Technology, 2008, 61(3): 455-460. [6] HE Y, HUANG L, SONG B, et al. Defluorination by ion exchange of SO42- on alumina surface: Adsorption mechanism and kinetics[J]. Chemosphere, 2021, 273: 129678. doi: 10.1016/j.chemosphere.2021.129678 [7] 张威, 杨胜科, 费晓华. 反渗透技术去除地下水中氟的方法[J]. 长安大学学报:自然科学版, 2002, 22(6): 3. [8] GUZMAN A, NAVA J, CORENO O, et al. Arsenic and fluoride removal from groundwater by electrocoagulation using a continuous filter-press reactor[J]. Chemosphere, 2016, 144: 2113-2120. doi: 10.1016/j.chemosphere.2015.10.108 [9] MEDELLIN-CASTILLO N A, LEYVA-RAMOS R, PADILLA-ORTEGA E, et al. Adsorption capacity of bone char for removing fluoride from water solution. Role of hydroxyapatite content, adsorption mechanism and competing anions[J]. Journal of Industrial & Engineering Chemistry, 2014, 20(6): 4014-4021. [10] 刘锐平. 饮用水氟污染控制原理与技术[J]. 应用生态学报, 2019, 30(1): 30-36. doi: 10.13287/j.1001-9332.201901.005 [11] FENG Q, LI T, QIAN B, et al. Chemical characteristics and utilization of coal mine drainage in China[J]. Mine Water and the Environment, 2014, 33(3): 276-286. doi: 10.1007/s10230-014-0271-y [12] 高宝玉, 岳钦艳, 黄红杉. 水处理无机混凝剂的研究进展[J]. 油气田环境保护, 1998, 8(2): 3. [13] 李为兵, 金雪中, 张红春, 等. 处理低温低浊水的混凝剂优选[J]. 中国给水排水, 2006, 22(13): 4. doi: 10.3321/j.issn:1000-4602.2006.13.013 [14] PARTHASARATHY N, BUFFLE J. Study of polymeric aluminium(III) hydroxide solutions for application in waste water treatment. Properties of the polymer and optimal conditions of preparation[J]. Water Research, 1985, 19(1): 25-36. doi: 10.1016/0043-1354(85)90319-7 [15] TANG H, LUAN Z. Features and mechanism for coagulation - flocculation processes of polyaluminum chloride[J]. Journal of Environmental Sciences, 1995, 15(2): 204-211. [16] KIMBERLY A G, YAO C H, CHARLES R O. Inorganic metal polymers: Preparation and characteriZation[J]. Journal of the American Chemical Society, 1995(4): 36-46. [17] KRAMER R W, KUJAWINSKI E B, HATCHER P G. Identification of black carbon derived structures in a volcanic ash soil humic acid by fourier transform ion cyclotron resonance mass spectrometry[J]. Environmental Science & Technology, 2004, 38: 3387-3395. [18] STOJANOVIC B J. Humic substances in the environment[J]. Journal of Environment Quality, 1974. [19] WANG X, XU H, WANG D. Mechanism of fluoride removal by AlCl3 and Al13: The role of aluminum speciation[J]. Journal of Hazardous Materials, 2020: 398. [20] SONG J, JIN P, JIN X, et al. Synergistic effects of various in situ hydrolyZed aluminum species for the removal of humic acid[J]. Water Research, 2019, 148: 106-114. doi: 10.1016/j.watres.2018.10.039 [21] 王亚军, 王进喜. 响应面法优化腐殖酸去除水中重金属铬的吸附条件及热力学研究[J]. 环境化学, 2013, 32(12): 2282-2289. doi: 10.7524/j.issn.0254-6108.2013.12.009 [22] RADIAN A, MISHAEL Y. Effect of humic acid on pyrene removal from water by polycation-clay mineral composites and activated carbon[J]. Environmental Science & Technology, 2012, 46(11): 6228-6235. [23] SHI B, LI G, WANG D, et al. Separation of Al13 from polyaluminum chloride by sulfate precipitation and nitrate metathesis[J]. Separation and Purification Technology, 2007, 54(1): 88-95. doi: 10.1016/j.seppur.2006.08.011 [24] WANG D, SUN W, XU Y, et al. Speciation stability of inorganic polymer flocculant–PACl[J]. Colloids and Surfaces A:Physicochem. Eng. Aspects, 2004, 243(1-3): 1-10. doi: 10.1016/j.colsurfa.2004.04.073 [25] 王霄. 基于羟基铝的混凝吸附除氟材料及机理研究[D]. 北京: 中国科学院大学, 2017. [26] PERNITSKY D J, EDZWALD J K. Solubility of polyaluminum coagulants[J]. Water Supply:Research and Technology-AQUA, 2003, 52(6): 395-406. doi: 10.2166/aqua.2003.0036 [27] WANG X, XU H, WANG D S. Mechanism of fluoride removal by AlCl3 and Al13: The role of aluminum speciation[J]. Journal of Hazardous Materials, 2020, 398: 122987. doi: 10.1016/j.jhazmat.2020.122987 [28] 刘艳静, 徐慧, 朱利军, 等. 不同有机物对混凝过程和余铝的影响[J]. 环境工程学报, 2015, 9(6): 2660-2666. doi: 10.12030/j.cjee.20150620 [29] SCHULTEN H R, LEINWEBER P. New insights into organic-mineral particles: Composition, properties and models of molecular structure[J]. Biology & Fertility of Soils, 2000, 30(5/6): 399-432. [30] SCHULTEN H R, SCHNITZER M. A state of the art structural concept for humic substances[J], 1993, 80(1): 29-30. [31] 孙鹏, 童庆, 象豫, 等. 水中腐殖酸对Al13混凝除氟的影响[J]. 环境工程学报, 2022, 16(1): 143-153. doi: 10.12030/j.cjee.202109152 [32] KAZPARD V, LARTIGES B S, FROCHOT C, et al. Fate of coagulant species and conformational effects during the aggregation of a model of a humic substance with All3 polycations[J]. Water Research, 2006, 40(10): 1965-1974. doi: 10.1016/j.watres.2006.03.014 [33] 胡磊鑫. 含铁氧化物活化过硫酸盐氧化降解典型有机污染物的研究[D]. 济南: 济南大学, 2021. [34] WEI J, GAO B, YUE Q, et al. Comparison of coagulation behavior and floc structure characteristic of different polyferric-cationic polymer dual-coagulants in humic acid solution[J]. Water Research, 2009, 43(3): 724-32. doi: 10.1016/j.watres.2008.11.004 期刊类型引用(3)
1. 燕佳妮,晁雷. 功能性除氟材料在废水处理中的研究进展及应用. 供水技术. 2025(02): 6-9 .  百度学术
百度学术
2. 邹志鑫,李敏,任晓影,刘佳惠,焦桂萍,赵瀛. Mg-Al水滑石的制备及对水中氟离子的吸附效果研究. 功能材料. 2024(04): 4179-4184+4200 .  百度学术
百度学术
3. 黄家榜,周锐,桑建伟,杨宏星,朱守诚. 新型除氟剂对含氟污水深度除氟的效果研究. 安徽化工. 2024(05): 123-125 .  百度学术
百度学术
其他类型引用(0)
-






 DownLoad:
DownLoad: