-
全程自养脱氮(completely autotrophic nitrogen removal over nitrite, CANON)工艺将短程硝化和厌氧氨氧化(anaerobic ammonium oxidation, ANAMMOX)2个过程置于同一反应器内进行[1]。相对于传统生物脱氮技术,CANON工艺具有无需外部碳源、节省曝气量和污泥产率低的显著优势[2]。在CANON工艺中,氨氧化细菌(ammonia oxidizing bacteria, AOB)与ANAMMOX菌起主要作用,AOB以O2为电子受体,将NH4+-N氧化成NO2−-N;ANAMMOX菌以NO2−-N为电子受体,将剩余NH4+-N氧化成N2,并生成少量的NO3−-N[3]。由于ANAMMOX所需要的NO2−-N是由短程硝化提供的,因此,稳定的短程硝化是CANON工艺稳定运行的必要条件。然而,由于亚硝酸盐氧化菌(nitrite oxidizing bacteria, NOB)与AOB生长所需的环境条件较为相似,系统中往往同时存在有AOB与NOB,若NOB过量增殖,NO2−-N被氧化为NO3−-N,则会导致短程硝化被破坏。
有诸多因素会对短程硝化造成影响,如pH、温度、溶解氧(dissolved oxygen, DO)和游离氨(free ammonia, FA)等[4]。其中,DO是控制CANON工艺中短程硝化和ANAMMOX稳定实现的关键参数。曝气过量使得DO质量浓度偏高,则会造成NOB的增殖和对ANAMMOX菌的抑制作用;曝气不足使得DO质量浓度偏低,则会使AOB活性受到抑制,氨氧化速率随之下降,ANAMMOX菌无法获得充足的NO2−-N基质进而导致脱氮效率降低[5-6]。李冬等[7]在序批式反应器(sequencing batch reactor, SBR)内构建了CANON工艺,结果表明,在DO为0.05~0.10 mg∙L−1的反应器中,ANAMMOX菌的活性不受影响,并在DO为0~0.40 mg∙L−1内通过增大曝气量提高总氮去除率;当DO达到0.5 mg∙L−1时,ANAMMOX菌的活性受到抑制,导致CANON工艺被破坏,但却实现了稳定的短程硝化,亚氮积累率达到93.35%。王会芳等[8]在以陶粒作为填料,研究了DO对生物膜CANON反应器的影响时发现,当DO超过1.75 mg∙L−1时,反应器中ΔNO3−-N/ΔTN值为0.239,严重偏离了理论值0.127,表明短程硝化遭到了严重破坏。JOSS等[9]在采用CANON工艺的污水处理厂中发现,由于2 h的过量曝气,DO质量浓度达到1 mg∙L−1以上,造成了对ANAMMOX菌活性的抑制,NO2−-N也开始积累,DO和NO2−-N质量浓度同时升高使得NOB在反应器内大量增殖,CANON反应器出水NO3−-N质量浓度从低于15 mg∙L−1升高到200 mg∙L−1以上,短程硝化和ANAMMOX均被破坏。
上述研究主要针对的是DO质量浓度对CANON工艺中短程硝化和ANAMMOX运行稳定性的破坏,但就CANON反应器中短程硝化运行失稳后调控恢复的相关报道较少。其中短程硝化过程的破坏主要表现为系统ΔNO3−-N/ΔTN值与理论值0.127的偏离,即系统中NOB开始发挥作用,ΔNO3−-N增加的同时,ΔTN也会由于NOB与ANAMMOX菌对NO2--N底物的竞争而减少,导致ΔNO3−-N/ΔTN值偏大。且由于采用的反应器形式不同以及污泥特性等方面的差异,研究者们对于CANON工艺中最佳DO质量浓度以及DO对CANON工艺中功能菌活性的影响还并未形成统一认识。本研究以CANON工艺中遭破坏的短程硝化为主要研究对象,通过限氧的方式对NOB活性进行抑制,从而恢复短程硝化,同步考察了NOB与ANAMMOX菌活性的变化,探究了DO质量浓度对CANON工艺中短程硝化恢复过程的影响。
-
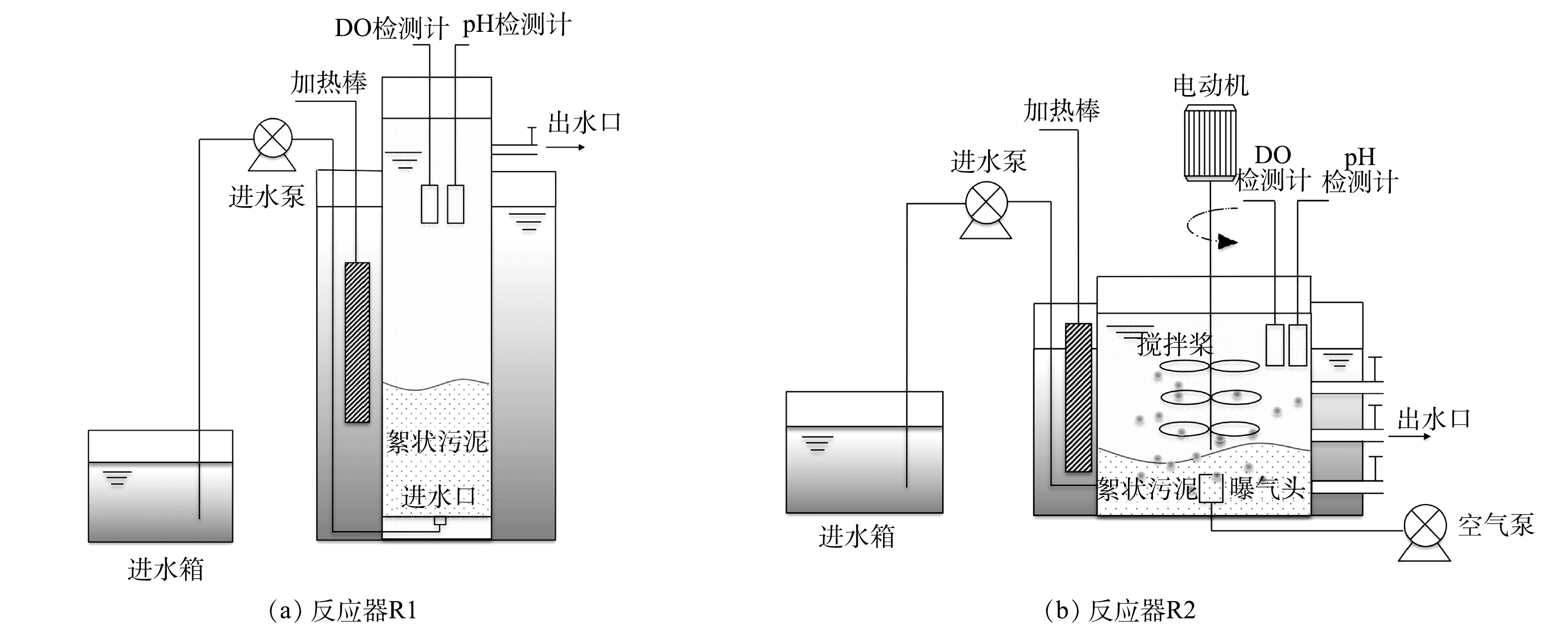
实验的不同阶段在不同的反应器中进行。其中,在连续流反应器R1中进行抑制期短程硝化恢复实验,而后,为保证曝气阶段的活性污泥混合反应完全,并避免污泥流失,改用序批式反应器R2进行过渡期和好氧期的短程硝化恢复实验。
连续流反应器R1由圆柱状有机玻璃制成,其内径为10 cm,高度为1.2 m,有效容积为8 L。反应器采用底部进水,上部出水的形式;R2反应器由圆柱状有机玻璃制成,其内径和高度分别为20 cm和27 cm,有效容积为5 L。反应器内部设有曝气及搅拌装置,外部通过水浴放置加热棒维持温度恒定。反应器每日运行6个周期,每周期总时长为4 h,其中进水10 min,反应200 min,沉淀20 min,出水10 min,由定时器自动控制各个装置的开关运行。排水比为50%。装置如图1所示。
-
各反应器进水均为无机人工配水,采用自来水进行配制,并采用NH4Cl、NaNO2或二者以一定比例混合作为主要氮源,添加适量的NaHCO3、NaH2PO4以及1 mL·L−1微量元素浓缩液Ⅰ和Ⅱ[10]。
反应器R1、R2中污泥均接种自课题组现有中试MBBR中短程硝化遭破坏的CANON工艺污泥。中试MBBR运行期间的ΔNO3−-N/ΔTN平均值为0.274,该值反映的是CANON工艺中的短程硝化效果[11],显著大于理论值,表明短程硝化性能较差。
-
取课题组现有中试MBBR当中的絮状污泥至反应器R1中,1~73 d内按照1:1.32的比例在进水中添加NH4+-N与NO2−-N强化ANAMMOX菌活性,初始进水流量设定为0.22 L∙h−1,即水力停留时间(hydraulic retention time, HRT)约为36 h,在实验初期采用较长的HRT有利于ANAMMOX菌的增长和持留[12]。同时采取密封措施,减少空气中的氧气进入,营造ANAMMOX菌的适宜生长环境,并对NOB的活性进行抑制。第74天时将反应器R1中污泥转移至反应器R2中,该过程中未对反应器中的污泥进行淘洗,尽量保持了污泥的原有形态和数量,避免了对实验的影响,并在此阶段逐步增加曝气量以及减少进水NO2−-N质量浓度以强化AOB的活性,使反应器由ANAMMOX工艺逐步转化为CANON工艺。反应器共连续运行130 d,运行工况如表1所示。
分别对连续运行第1天和第80天的污泥进行高通量测序,考察微生物群落结构的变化规律,并在连续运行的第1、28、36、48、58、90以及110天进行批次实验测定ANAMMOX菌以及NOB活性。
-
1) NH4+-N使用纳氏试剂分光光度法检测;NO2−-N使用N-(1-萘基)-乙二铵光度法检测;NO3−-N使用紫外分光光度法检测;pH使用METTLER TOLEDO F2 基础型便携式pH计检测;温度和DO使用METTLER TOLEDO FG4基础型便携式溶氧仪检测。TN= NH4+-N+NO2−-N+NO3−-N [8]。FA质量浓度按式(1)计算。
式中:
CNH+4-N 为混合液中NH4+-N质量浓度,mg∙L−1;T为混合液温度,℃。2)活性测定。以TN比降解速率q作为ANAMMOX活性指标,根据式(2)进行计算。
式中:ΔTN为反应过程中TN质量浓度变化量,mg∙L−1;t为反应时间,h;M为混合液挥发性悬浮固体质量浓度,g∙m−3。
采用NOB的比耗氧速率(specific oxygen uptake rate, SOUR)r表征NOB活性[14],根据式(3)进行计算。
式中:D1为不添加底物时单位实验时间的DO质量浓度差,mg·(L·h)−1;D2为添加NO2−-N底物时单位实验时间的DO质量浓度差,mg·(L·h) -1。
3)微生物分析。委托上海生工生物工程有限公司进行高通量测序,对污泥样品进行宏基因组16S rDNA测序,分析微生物群落结构[15]。
-
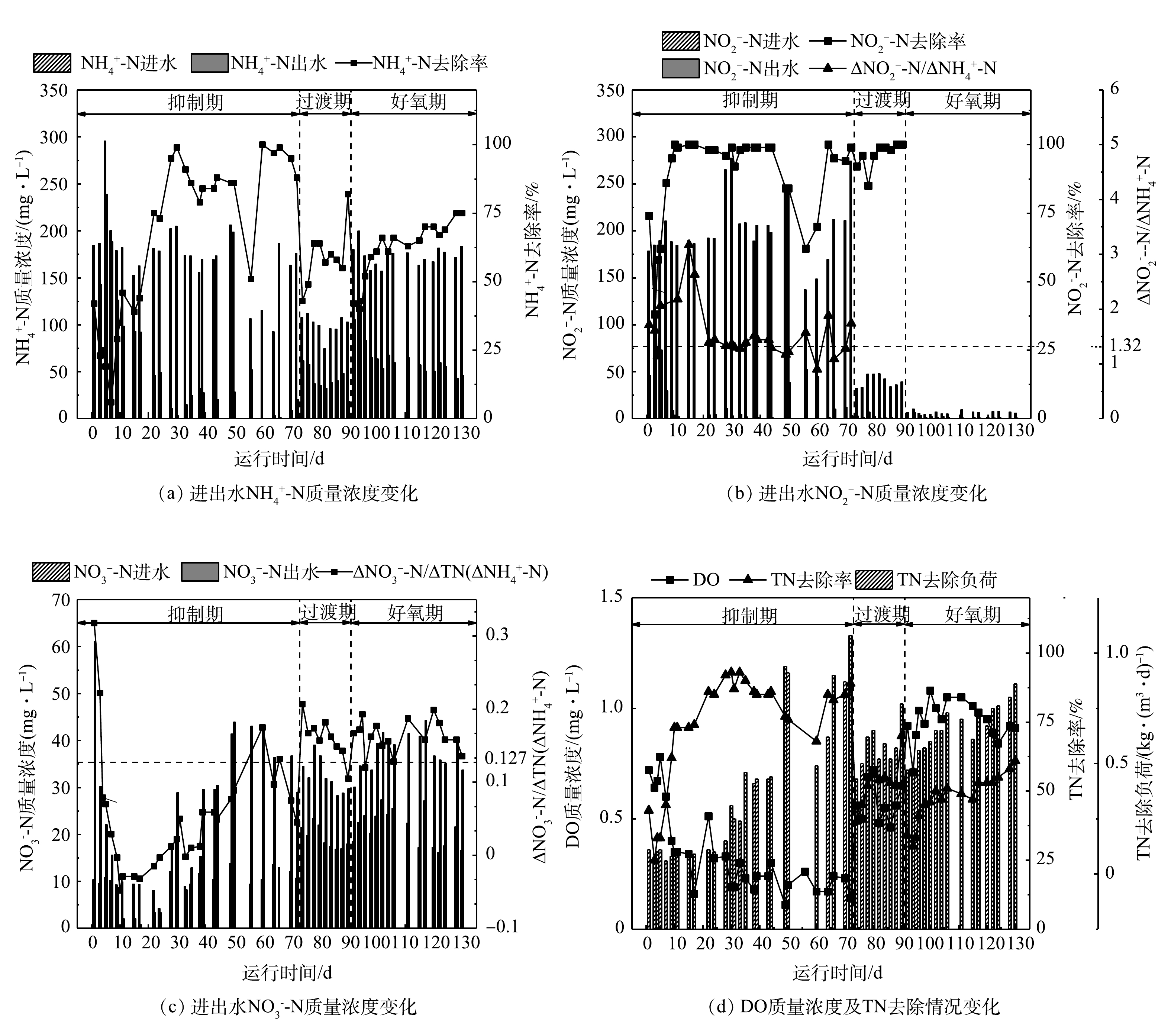
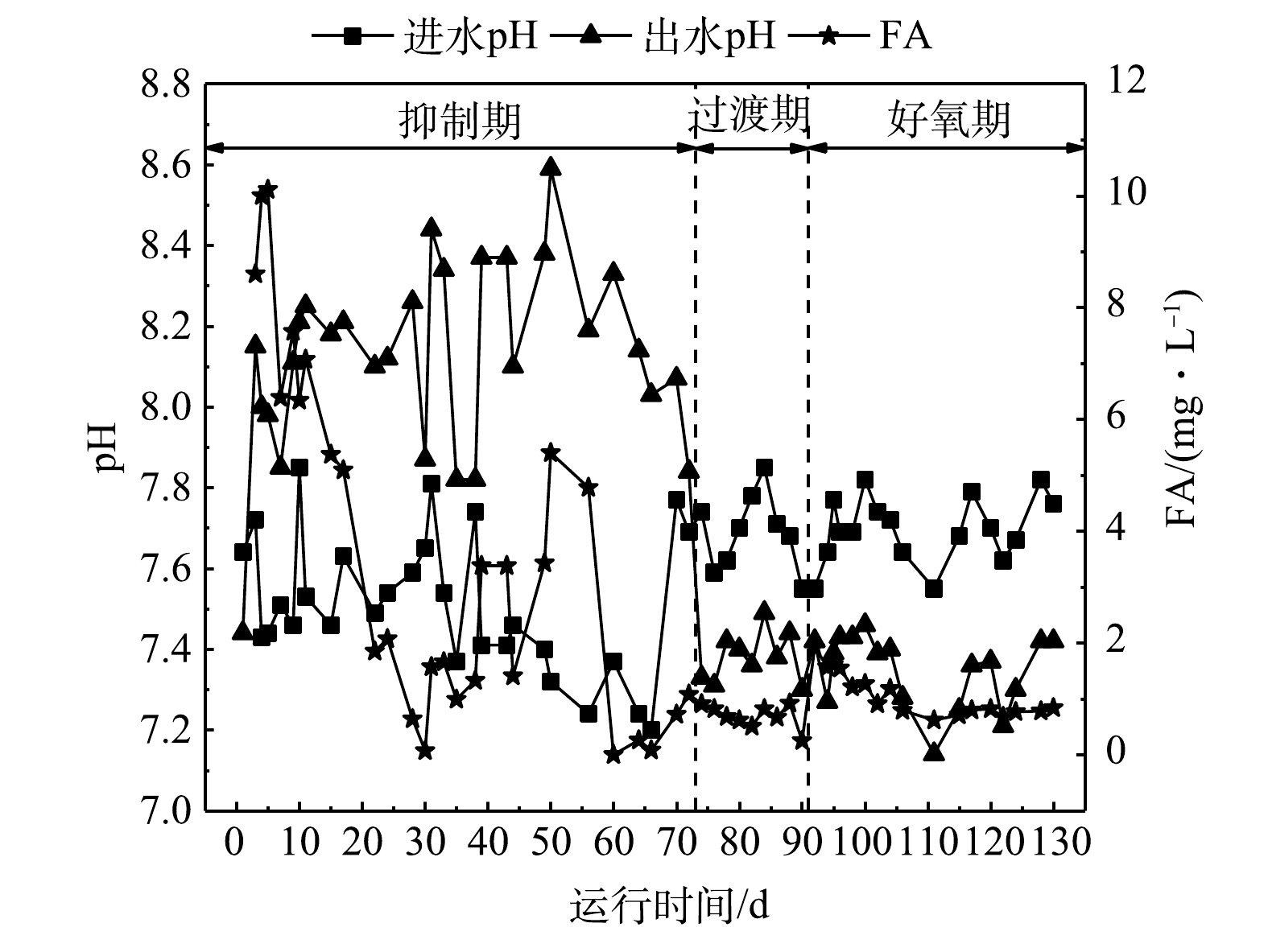
1)抑制期。反应器共连续运行130 d。如图2(a)所示,在抑制初期0~3 d,NH4+-N去除率下降,NH4+-N的平均出水质量浓度为124.6 mg∙L−1。由图2(b)可见,NO2−-N去除率由74.3%降低至38.1%,ΔNO2−-N/ΔNH4+-N值大于ANAMMOX特征理论值1.32[8]。这表明此时ANAMMOX作用不明显。由于运行初期的进水流量相对较大,初始进水流量为0.22 L∙h−1,且未对进水中的DO进行吹脱,反应器内DO高于0.6 mg∙L−1,超过了ANAMMOX菌对DO的最适质量浓度0.15~0.30 mg∙L−1 [16]。此外,如图3所示,运行初期FA质量浓度接近10 mg∙L−1。有研究表明,该水平FA质量浓度会对ANAMMOX菌的活性存在一定的抑制作用[17]。如图2(c)和图2(d)所示,0~3 d内的ΔNO3−-N/ΔTN值均高于理论值0.127,出水NO3−-N质量浓度偏高,TN去除率由42.7%下降至24.5%。这表明此时短程硝化效果不理想,反应器内主要进行的是AOB以及NOB利用进水中的DO进行氨氧化以及亚硝酸盐氧化反应。
在第7天将进水流量减半至0.11 L∙h−1,由于AOB和NOB持续消耗进水中的DO进行硝化反应,导致DO质量浓度在第28天下降至0.33 mg∙L−1。在此阶段,由图2(a)和图2(b)可见,NH4+-N和NO2−-N虽均有不同程度的去除,但NH4+-N平均去除率仅为49.0%,NO2−-N去除率则稳定在95.0%以上,ΔNO2−-N/ΔNH4+-N平均值为2.02,远高于ANAMMOX反应计量理论值1.32。这表明ANAMMOX尚未成为反应器内的主要反应。此外,如图2(c)所示,ΔNO3−-N/ΔTN值小于0,远低于ANAMMOX反应计量理论值0.127。由图2(d)可见,TN去除率有所上升。由于本研究采用的是无机配水,排除了反硝化细菌利用外源有机物进行反硝化脱氮的因素。其原因可能为,DO缺乏导致部分好氧菌因底物匮乏死亡,之后转化为可降解有机物,反硝化菌利用其作为碳源进行内源呼吸代谢[18]。
在第30天,将进水流量还原至初始进水流量0.22 L∙h−1。如图2(a)~(d)所示,当反应器内DO质量浓度降低至0.19 mg∙L−1时,NH4+-N和NO2−-N去除率分别升高至98.7%和98.9%,ΔNO3−-N/ΔTN由负值开始升高,TN去除率达到93.1%,此时的ΔNO2−-N/ΔNH4+-N值为1.36,与理论值1.32相比稍有偏高。这表明NH4+-N与NO2−-N基本实现同步去除,ANAMMOX作用得到提升,但反应器内依然存在硝化反硝化作用。
在31~73 d,分别于第35天以及第49天将进水流量提高至初始进水流量的2倍(0.44 L∙h−1)和4倍(0.88 L∙h−1)。如图2(b)和图2(d)可知,ΔNO2−-N/ΔNH4+-N平均值为1.37,TN去除负荷升高至1.08 kg∙(m3∙d)−1。这表明ANAMMOX菌活性得到提升,ANAMMOX占主导作用,但偏高的ΔNO2−-N/ΔNH4+-N值说明系统中依旧存在NOB参与的亚硝酸盐氧化,本阶段较低的DO并不能完全抑制NOB,若要进一步淘汰系统中的NOB,可能需要更严格的限氧条件。同时,由图2(c)可以看出,ΔNO3−-N/ΔTN值继续升高向理论值趋近,但仍低于0.127。这表明仍有死亡微生物作为可生物降解有机物参与反硝化过程,异养菌同NOB对DO的竞争,使得NOB难以利用有限的DO进行亚硝酸盐氧化反应,有利于对短程硝化的进一步恢复。
2)过渡期。在此阶段,进水NH4+-N质量浓度由150 mg∙L−1降低至125 mg∙L−1;进水NO2−-N质量浓度由200 mg∙L−1降低至50 mg∙L−1。因ANAMMOX反应基质NO2−-N减少,第74天时,由图2(a)和图2(d)可见,NH4+-N和TN去除率偏低,分别为42.8%和39.9%。通过过渡期恢复曝气,AOB活性预期可逐步恢复,以保证NO2−-N来源,第91天时,NH4+-N和TN去除率分别上升至82.4%和70.0%。此外,由于ΔNH4+-N和ΔNO2−-N反映的是AOB与ANAMMOX菌共同作用的结果,不宜继续采用ΔNO2−-N/ΔNH4+-N作为衡量指标[11]。由图2(c)可见,过渡期的出水NO3−-N质量浓度增长量较抑制期减小,同时,ΔNO3−-N/ΔTN值由过渡初期时0.207下降至0.105。这说明,由于ANAMMOX菌的活性得到强化,NOB难以同ANAMMOX菌竞争NO2−-N,同时,由于DO限制,AOB较好的溶解氧亲和能力使其在与NOB竞争DO的过程中获得优势,短程硝化效果得到改善。
3)好氧期。在此阶段,进水NH4+-N质量浓度由125 mg∙L−1提高至200 mg∙L−1;进水NO2−-N质量浓度由50 mg∙L−1降低至0。由于NO2−-N质量浓度的进一步限制,在第92天时,由图2(a)和图2(d)可见,NH4+-N和TN去除率较前一阶段急剧下降。随后,NH4+-N和TN去除率逐渐得到提升。根据VAN DER STAR等[19]的研究结果,NOB对NO2−-N的半饱和常数为12~955 μmol∙L−1,而ANAMMOX菌对NO2−-N的半饱和常数为0.2~3 μmol∙L−1,即ANAMMOX菌对NO2−-N的亲和能力更强,从而在NO2−-N质量浓度较低时能够先于NOB将其利用。此外,随着AOB活性在好氧期的增强,氨氧化对系统中DO的消耗,进一步抑制了NOB的活性,并提升了ANAMMOX的脱氮效果。运行第130天,如图2(a)~(d)所示,NH4+-N去除率升高至75.0%,NO2−-N去除率维持较高水平,TN去除负荷达到0.86 kg∙(m3∙d)−1,ΔNO3−-N/ΔTN值为0.136,较理论值(0.127)偏高。此结果说明NOB仍有一定活性,系统中的NOB没有被完全淘汰。
-
ANAMMOX菌活性批次实验进水NH4+-N和NO2−-N质量浓度分别为43 mg∙L−1和57 mg∙L−1;NOB活性批次实验进水NH4+-N和NO2−-N质量浓度分别为0和50 mg∙L−1。如图4所示,ANAMMOX菌在限氧抑制初期活性恢复较缓慢,此时,污泥处于对反应器内DO质量浓度波动式下降的适应阶段。此后,ANAMMOX菌活性迅速升高,由0.11 kg∙(kg∙d)−1(以VSS计)升高至0.34 kg∙(kg∙d)−1,但NOB的SOUR值自抑制期开始始终呈下降趋势,由第1天的1.68 kg∙(kg∙d)−1(以VSS计)降低至第110天的0.57 kg∙(kg∙d)−1,说明抑制效果显著。在本研究中,在污泥以絮状污泥为主且未形成明显颗粒污泥的条件下,ANAMMOX菌活性依然得到了强化,仅在当反应器进入过渡期恢复曝气后,由于好氧条件的限制,ANAMMOX菌活性开始下降,于第110天下降至0.26 kg∙(kg∙d)−1,但与反应初期的0.08 kg∙(kg∙d)−1相比,ANAMMOX菌活性仍有较大提升。若在后续长期运行中形成颗粒污泥,或许可进一步提升ANAMMOX菌的活性。此外,当解除限氧抑制恢复曝气后,NOB活性并未出现回升趋势,但直至反应运行的第110天,NOB活性也并未完全丧失,表明限氧条件虽然能够有效抑制NOB的活性,但不能彻底淘汰NOB,并且在后续长期运行过程中,存在NOB逐渐克服抑制作用进行增殖从而再次使短程硝化遭到破坏的风险。
-
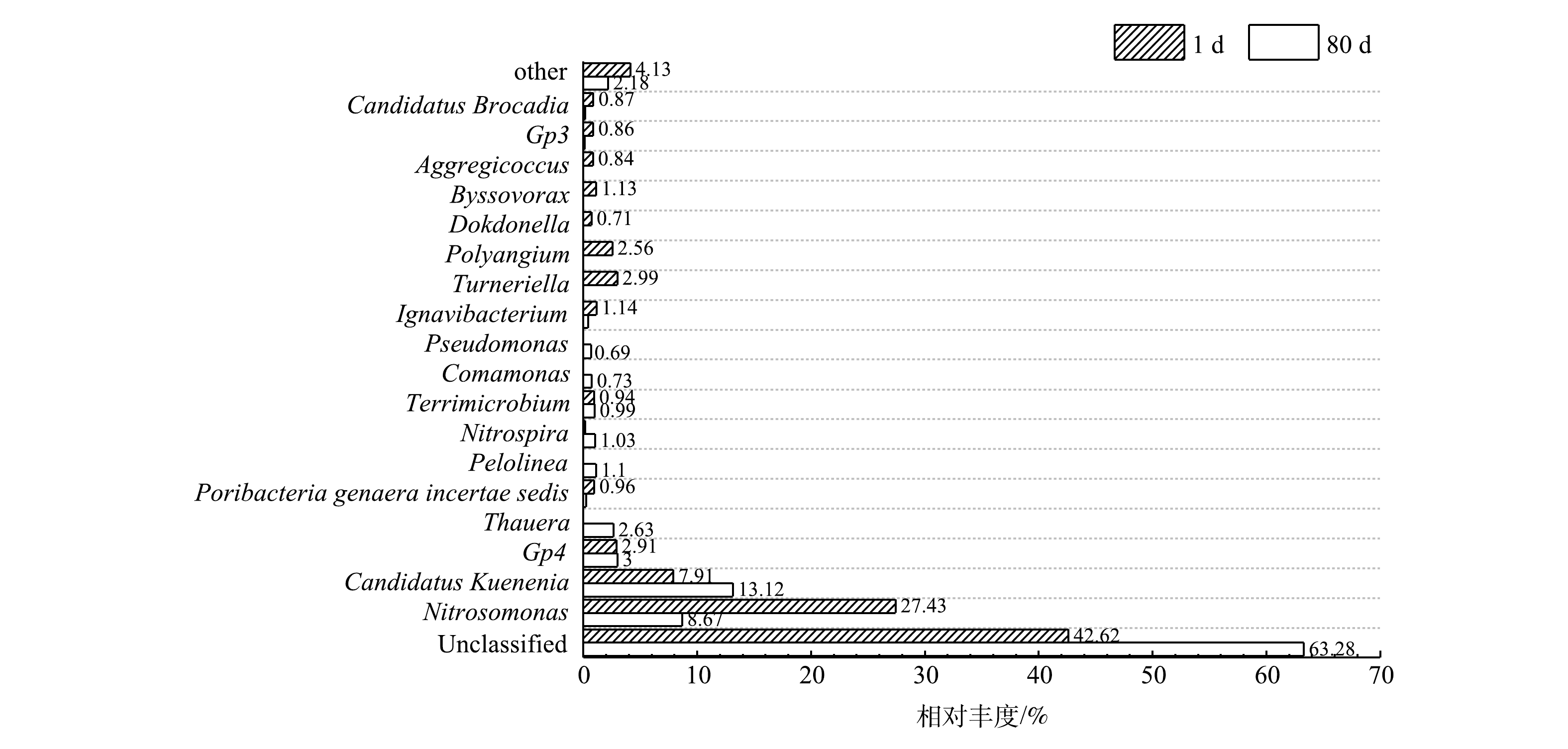
分别取连续运行第1天和第80天反应器中污泥进行高通量测序,抑制期前后系统中的微生物群落结构变化规律如图5所示。短程硝化恢复过程中,检测到的ANAMMOX菌有Candidatus Kuenenia和Candidatus Brocadia。其中,Candidatus Kuenenia为优势菌属,其丰度由7.91%增长至13.12%。有研究表明,Candidatus Brocadia适于在严格厌氧环境下生长,而Candidatus Kuenenia则可以接受混合液中存在少量的溶解氧[16],表明抑制期的低DO质量浓度可促进Candidatus Kuenenia的生长。同时,反应器中存在少量的Candidatus Brocadia,其丰度在抑制前的絮状污泥中为0.87%,经过过渡期的NO2−-N质量浓度波动后,丰度降至0.01%以下。有研究表明,在SBR中对Candidatus Kuenenia和Candidatus Brocadia的选择可能是基于对限制性底物(如NO2−-N)的亲和力,Candidatus Kuenenia对NO2−-N的亲和性能较强[19]。因此,在本实验有限的进水NO2−-N质量浓度条件下,Candidatus Kuenenia占据了优势生长地位。AOB中的优势菌属为Nitrosomonas,由于抑制期的低DO质量浓度,导致AOB的生长受到抑制,相对丰度由27.43%降低至8.67%。NOB的主要菌属为Nitrospira,NOB在抑制期前后的特征较为一致,即在微生物群体中的相对丰度较低,平均低于AOB以及ANAMMOX菌一个数量级。其中,经抑制后的污泥中Nitrospira相对丰度由抑制前的0.01%以下升高至1.03%,即NOB相对丰度经过限氧抑制后反而上升。
此外,还检测到几种反硝化菌属,相对丰度均在抑制期后有所提高,如Thauera、Pseudomonas和Comamonas,均被证明具有利用有机物为碳源进行反硝化的能力[20-22],由于进水中未添加有机物,因此,这进一步印证了抑制期间可能发生的内源反硝化过程。
-
本研究中,Nitrospira为检测出的主要NOB菌属,这与传统污水处理硝化菌种一致[23]。经过抑制期的短程硝化恢复,活性批次实验结果表明NOB活性抑制情况良好,然而,丰度却有所升高。可见,NOB的活性和生物量的变化趋势并不同步。类似的结果也出现在课题组同期进行的SBR间歇曝气恢复的短程硝化实验中,反应器恢复短程硝化后,AOB活性稳定的同时其丰度显著下降,而NOB虽检测不到活性,但其相对丰度呈上升趋势[24]。
普遍认为,AOB的氧半饱和常数小于NOB[25],在低DO条件下,AOB的生长速率几乎不受影响,而 NOB的生长速率显著降低。然而,杨庆等[26]对此的研究表明,低DO系统内AOB和NOB的氧半饱和常数分别为0.281 mg∙L−1和0.280 mg∙L−1,两者非常接近,并且NOB的生长速率远大于AOB。结合分析本实验中AOB和NOB丰度的变化,推测其原因可能为:1)抑制期低DO质量浓度可限制AOB的生长和氨氧化反应速率,生长速率较NOB缓慢,导致AOB相对丰度降低;2)尽管限氧条件抑制了NOB的活性,但抑制期进水NO2−-N质量浓度较高,充足的底物质量浓度为NOB在不利条件下生长提供了可能性,因此NOB相对丰度升高;3)在抑制期间反应器不能做到与空气完全隔绝,难免有氧气进入反应器,且实验进水为DO未经吹脱的自来水人工配制,因此,反应器内DO会从实验进水中得到一定补充,导致NOB仍可利用DO进行增殖;4)本实验进水为无机配水,不含外源有机物,因此,减弱了异养菌同NOB对DO的竞争作用,进一步提升了NOB利用DO进行增殖的空间。
USHIKI等[27]对NOB基因组的分析结果表明:Nitrospira含有的编码细胞色素氧化酶bd基因,能够在低氧条件下被激活,使细菌在限氧环境下也能够完成细胞生长的过程。张亮等[28]认为,当受到抑制时,NOB或可通过提高丰度等方式克服抑制作用。可见,NOB仍有部分活性规律尚未明确,这些特性均给CANON工艺中针对NOB的抑制带来了挑战。
值得一提的是,新加坡樟宜再生水厂地处热带,污水温度在(30
± 2)℃,适合ANAMMOX菌的增殖并有利于实现短程硝化,但其在由测流向主流ANAMMOX工艺转换的过程中,同样存在对NOB抑制的问题[29]。一方面,侧流工艺中的高NH4+-N质量浓度(500~1 500 mg∙L−1)使得FA质量浓度高于抑制NOB的阈值(0.08~0.82 mg∙L−1)[30],而主流工艺中城市污水较低的NH4+-N质量浓度(30~100 mg∙L−1)使得NOB抑制较为困难;另一方面,AOB、NOB及ANAMMOX的活性-温度系数在不同温度下的差异较大[31],主流工艺中季节变换导致的城市污水温度有波动,使得污泥微生物种群和数量发生改变,同样对NOB的抑制以及ANAMMOX菌的持留带来挑战。通过对本研究及樟宜再生水厂在以上两方面运行条件的对比,可以在一定程度上解释本研究中NOB无法被完全抑制的原因:一是本研究中的NH4+-N质量浓度在长期运行过程中处于较低水平,因此,缺乏FA对NOB的持续抑制作用;二是本研究进水温度维持在25℃左右,与ANAMMOX菌的适宜中温条件有一定偏离。此外,针对NOB在系统中的相对丰度变化,孙延芳等[32]关于絮体污泥龄(sludge retention time, SRT)对CANON工艺运行稳定性的影响研究中发现,不淘洗絮体污泥时,系统NOB丰度显著增加,并通过定量PCR技术分析得出,絮体SRT时间越长,ANAMMOX菌的丰度越高。因此,有必要在后续的长期运行中持续关注微生物的丰度变化及系统稳定性,结合控制合理污泥龄的手段来实现功能菌(AOB和ANAMMOX菌)的有效持留和NOB的淘洗。
-
1)经过抑制期的限氧,ANAMMOX活性由0.08 kg∙(kg∙d)−1上升至0.34 kg∙(kg∙d)−1,TN去除率可达88.6%,TN去除负荷最高可达1.08 kg∙(kg∙d)−1,ANAMMOX占主导作用。
2) Candidatus Kuenenia为ANAMMOX菌优势菌属,Nitrospira为NOB的主要菌属,经过抑制期恢复,二者的相对丰度分别由7.91%和低于0.01%增长至13.12%和1.03%。
3)通过短程硝化恢复,ΔNO3−-N/ΔTN值由0.318逐渐恢复至0.136,短程硝化基本恢复,但较理论值0.127稍有偏高,仍存在亚硝酸盐氧化过程。
4)连续运行期间,NOB活性降低显著,但相对丰度有所提高,于微生物群体中仍具有一定的相对丰度,在后续运行中有适应抑制作用并进行增殖的可能,CANON工艺短程硝化仍具有被破坏的风险。
CANON工艺中短程硝化恢复及ANAMMOX强化策略
Short-cut nitrification recovery and ANAMMOX enhancement strategy in CANON process
-
摘要: 为了对CANON工艺中遭破坏的短程硝化进行恢复,并对ANAMMOX菌的活性进行强化,在第1阶段(抑制期)采用连续流反应器,限制DO质量浓度为0.1~0.4 mg∙L−1,利用限氧条件对NOB活性进行抑制,投加NH4+-N和NO2−-N,经过73 d运行,ANAMMOX菌活性由0.08 kg∙(kg∙d)−1(以VSS计)上升至0.34 kg∙(kg∙d)−1;NOB比耗氧速率(SOUR)由1.68 kg∙(kg∙d)−1(以VSS计)降低至0.79 kg∙(kg∙d)−1,活性显著降低,系统TN去除率由42.7%升高至88.6%,NH4+-N和NO2−-N同步去除,ΔNO3−-N/ΔTN值向理论值0.127趋近。第2阶段(过渡期)、第3阶段(好氧期)采用SBR进行,分别将DO维持在0.4~0.7 mg∙L−1和0.7~1.0 mg∙L−1,至第130天,NOB活性降低至0.57 kg∙(kg∙d)−1,TN去除负荷达到0.86 kg∙(m3∙d)−1,ΔNO3−-N/ΔTN值由抑制前的0.318降低至0.136,短程硝化基本恢复。在短程硝化恢复过程中,ANAMMOX菌优势菌属Candidatus Kuenenia的相对丰度由7.91%增长至13.12%,但NOB的主要菌属Nitrospira的相对丰度由低于0.01%增至1.03%,表明在后续长期运行过程中,依然存在短程硝化遭到破坏的风险。Abstract: In order to recover the short-cut nitrification in CANON process and enhance the activity of anaerobic ammonium oxidation (ANAMMOX) bacteria, at the first stage (inhibition period), a continuous flow reactor was used and the dissolved oxygen (DO) concentration was limited to 0.1-0.4 mg∙L−1, so as to inhibit the activity of NOB by oxygen limitation. At the same time, NH4+-N and NO2−-N was added. After 73 days of operation, the activity of ANAMMOX bacteria increased from 0.08 kg∙(kg∙d)−1 (calculated as VSS) to 0.34 kg∙(kg∙d)−1. The specific oxygen uptake rate (SOUR) of nitrite oxidizing bacteria (NOB) decreased from 1.68 kg∙(kg∙d)−1 (calculated as VSS) to 0.79 kg∙(kg∙d)−1, indicating a significant decrease in the activity of NOB. The total nitrogen (TN) removal rate of the system increased from 42.7% to 88.6%, NH4+-N and NO2−-N were removed simultaneously, and the ΔNO3−-N/ΔTN value approached to the theoretical value of 0.127. The second stage (transition period) and the third stage (aerobic period) were carried out by SBR mode, and the DO concentration maintained at 0.4~0.7 mg∙L−1 and 0.7~1.0 mg∙L−1, respectively. On the 130th day, the activity of NOB decreased to 0.57 kg∙(kg∙d)−1, and the TN removal load reached 0.86 kg∙(m3∙d)−1. Meanwhile, the ΔNO3−-N/ΔTN value decreased from 0.318 before the inhibition to 0.136, and the short-cut nitrification was basically recovered. In the process of short-cut nitrification recovery, the relative abundance of the dominant ANAMMOX bacteria, Candidatus Kuenenia, increased from 7.91% to 13.12%, while the relative abundance of the main NOB bacteria, Nitrospira, increased from less than 0.01% to 1.03%, this indicated that a risk of short-cut nitrification destruction will still exist in the following long-term operation.
-
邻苯二甲酸酯(phthalate esters,PAEs)是邻苯二甲酸形成的酯的统称,近年来因其在塑料、建筑材料、个人护理品、食品包装和医疗产品中的广泛使用而受到越来越多的关注[1]. 由于PAEs与聚合物之间没有化学键合,因此很容易从产品中释放出来. 目前PAEs已经在多种环境基质中检测到,包括空气[2]、水[3]、土壤[4]和生物群体[5]. 作为典型的内分泌干扰物,PAEs具有致畸、致癌和致突变“三致”效应[6],长期接触会带来许多不良后果. 由于PAEs的广泛应用、大规模生产和对人类健康的不利影响,美国环境保护署(USEPA)已将邻苯二甲酸二甲酯(DMP)、邻苯二甲酸二乙酯(DEP)、邻苯二甲酸二丁酯(DBP)、邻苯二甲酸丁苄酯(BBP)、邻苯二甲酸(2-乙基己基)(DEHP)和邻苯二甲酸二辛酯(DnOP)列为6种“优先控制的有毒污染物”,随后我国环境保护局也将DMP、DBP和DnOP列入重点控制污染物黑名单[7].
土壤是重要的环境介质,也是污染物的主要储存库[8]. 近年来,PAEs在土壤中的污染越来越突出,在我国个别地区甚至达到mg·kg−1级别[9],通过食物链对人类健康构成风险,这使它们成为土壤中最受关注的有机污染物之一. 农用薄膜(棚膜和地膜)是我国农业土壤中PAEs污染的重要来源,随着设施农业规模的不断扩大,我国己成为世界上最大的农膜覆盖区[10]. 土壤中的PAEs还与农业实践活动有关,通过各种方式进入土壤环境,例如有机肥料,地表径流,废水灌溉和大气沉降等[11].
目前,国内外很多学者已经开始关注PAEs在土壤中的污染,这些研究涵盖了不同的耕地,包括塑料温室[8, 12]、菜地[13 − 14]、果园[13]、和农田作物[15]等等,然而大多数只分析了一种类型的土地. 农业实践类型受人类活动的影响,并决定了区域环境的植被覆盖和耕作方式,因此PAEs的含量可能与不同的农业类型有关. 事实上,农药、有机肥以及农膜的施用,土壤深度,土壤理化性质及周边污染源的影响均有可能导致不同类型土壤中PAEs的差异[13]. 目前少有关于农业土壤与PAEs污染综合分析的研究.
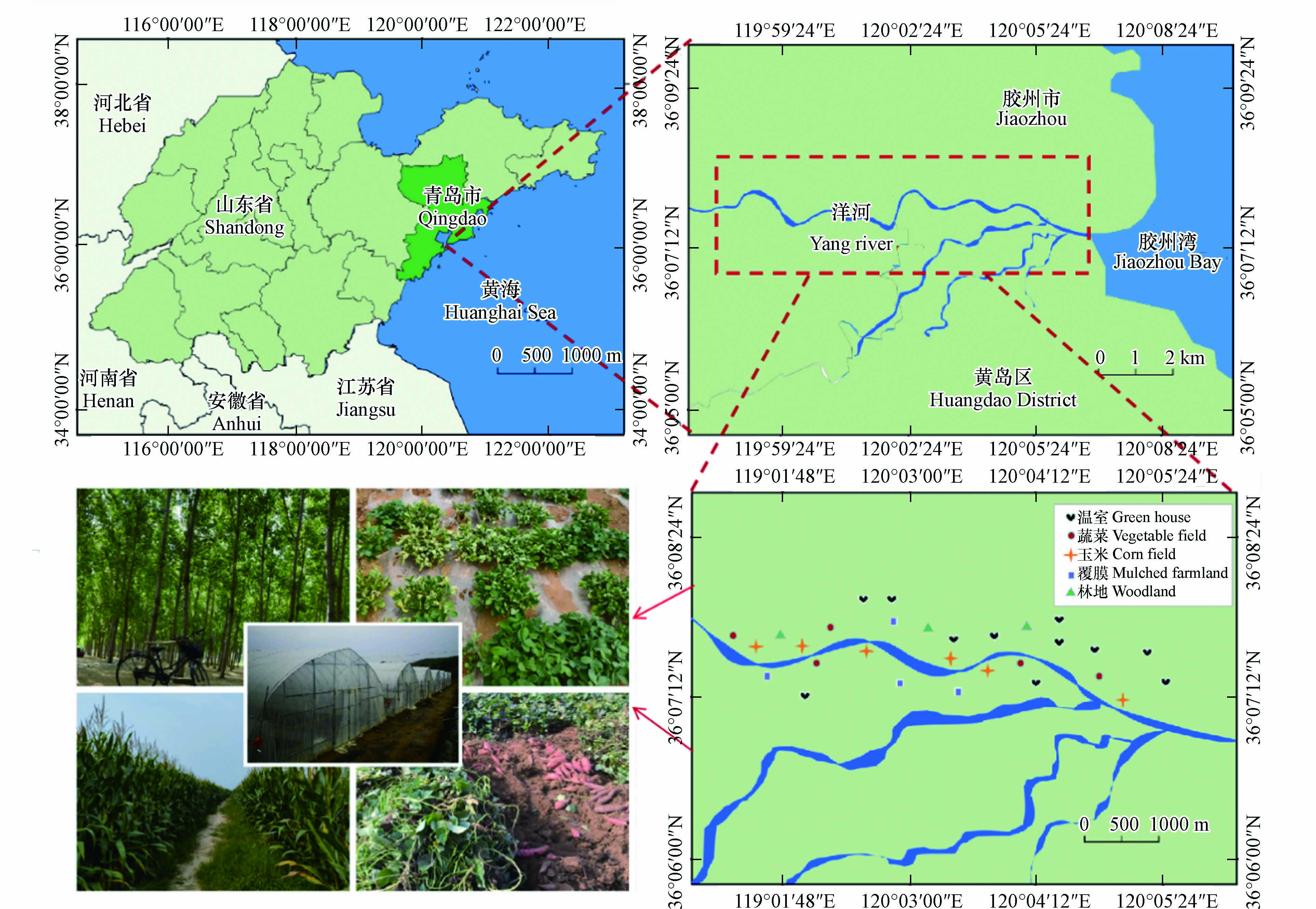
洋河位于中国北方的胶东半岛,发源于青岛市黄岛区北部,于胶州湾入海. 地处北温带季风区域,降雨主要发生在夏季. 河流两岸主要是村庄、农业区和产业园,农业基础雄厚,粮食生产和经济作物的种植占据主要地位. 同时也有其自身的特点,例如不同的作物种植和不同的覆盖类型. 农田土壤附近多有农药瓶、化肥包装袋、垃圾堆放和农膜残留等现象,“白色污染”不容小觑. 所有这些因素都可能对土壤中PAEs的残留产生影响,目前尚未有关于该地区土壤中PAEs污染的相关报道. 因此,本论文研究了洋河农业区不同实践类型的农业土壤中PAEs的分布模式以及土壤理化性质与PAEs的关系,进一步分析潜在的污染源,为建立农业生态系统中PAEs控制措施提供支持.
1. 材料与方法(Materials and methods)
1.1 样品采集
2021年9月,根据洋河周边的农业生产布局和种植类型(图1),共收集56份具有代表性的土壤样本,包括温室土地(n=22)、覆膜耕地(n=8)、玉米地(n=12)、蔬菜地(n=8)和林地(n=6).遵循五点采样法,每3份子样品形成1个复合样品,钢尺精确测量距离和深度,使用不锈钢铲采集土壤样品(0—10 cm、10—20 cm). 取样时去除枯枝落叶、大块石子以及破碎的塑料薄膜,远离植物根部和刚刚施肥的区域. 样品储存在预先清洗的棕色玻璃瓶中,于−50 ℃冷冻干燥,研磨过100目筛,储存在−4 ℃冰箱中待分析.
1.2 仪器与试剂
仪器:气质联用仪(日本岛津,GC-MS 2010Plus);冷冻干燥机(北京亚星,LGJ-10N);超声振荡仪(上海科号,SK5200H);台式离心机(奥豪斯,FC576);涡旋振荡器(上海精业,XW-80A);旋转蒸发仪(BUCHI,R-10);氮吹仪(Organomation Associates,N-EVAP111).
试剂:6种PAEs混合标准溶液(DMP、DEP、DBP、BBP、DEHP和DnOP)和内标物(磷酸三丁酯-d27、苯甲酸苄酯)浓度均为1000 µg·mL−1,购自美国AccuStandard公司;正己烷、丙酮和二氯甲烷均为色谱级,购自天津科密欧公司. 柱层析硅胶(100—200 目,青岛海洋化工厂)和氧化铝(100—200目,上海国药试剂)使用前分别在180 ℃和400 ℃活化4 h,冷却后加入5%去离子水;无水硫酸钠(分析纯,上海国药试剂)使用前在马弗炉450 ℃灼烧4 h.
1.3 样品处理
参考之前的文献方法[16],准确称取10.0 g样品置于玻璃离心管中,添加已知量的磷酸三丁酯-d27,加入20 mL正己烷-丙酮(1:1, V/V)涡旋混合,经两次超声(35 ℃,15 min)和离心(3000 r·min−1,10 min),合并上清液. 上清液经旋蒸浓缩后,转移至从下到上填充有中性氧化铝-硅胶-无水硫酸钠(高度分别为6 cm、12 cm和1 cm)的玻璃层析柱中,依次用20 mL正己烷、20 mL二氯甲烷-丙酮混合液(1:1, V/V)和20 mL乙酸乙酯洗脱. 氮气条件下吹干,加入已知量的苯甲酸苄酯,正己烷定容到200 µL.
同时分析了土壤样品的理化性质:土壤pH通过多参数水质检测仪(美国HACH)在土壤-水(1:2.5, W/V)提取中测量;元素分析仪(德国Elementar)用来测定土壤中的总有机碳(TOC)和总氮(TN).
1.4 GC-MS测定条件
色谱条件:使用GC-MS 2010Plus(日本岛津)和DB-5MS石英毛细管柱(30 m×0.25 mm×0.25 µm)进行色谱分离. 载气:高纯氮气;柱流量:1 mL·min−1;进样体积:1 µL,不分流进样;进样口和柱箱温度分别为250 ℃和50 ℃. 升温程序:初温50 °C,保持1 min,以15 °C·min−1的速率升温至200 °C,保持2 min;以8 ℃·min−1的速率升温至300 °C,保持8 min.
质谱条件:选择性离子监测为SCAN模式,离子源和传输线温度分别为230 ℃和280 ℃.
1.5 质量保证与控制
实验过程中不使用任何塑料制品,所有非计量玻璃器皿经纯水洗涤和有机溶剂淋洗后经马弗炉450 ℃灼烧4 h. 分析过程中,每12个样品进行一组程序空白、基质加标和样品平行样. 在程序空白中检测到少量DBP、DEHP,实际检测样品均经过空白校正. 将回收内标添加到所有样品中,回收率为81.21%±5.38%. 6种目标单体的回收率范围为78.32%—118.63%,相对标准偏差<10%. 仪器检出限定义为信噪比的3倍,范围为0.61—2.36 ng·L−1. 采用0.05、0.1、0.2、0.5、1、5、10、20 ng·L−1的6种PAEs绘制标曲,各物质标曲R2=0.995—0.999,满足要求.
1.6 健康风险评价
洋河农业区内居民的非致癌和致癌风险根据USPEA推荐的模型进行评估. DMP、DEP、DBP、和DnOP被认为是与人体健康相关的非致癌物质,DEHP和BBP是致癌物质. 当地居民主要通过饮食途径(食物摄入)和非饮食途径(土壤摄入、皮肤接触、呼吸)摄入PAEs. 公式如下:
stringUtils.convertMath(!{formula.content}) (1) stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) stringUtils.convertMath(!{formula.content}) (6) 其中,Csoil是土壤中目标单体的浓度(mg·kg−1);ADDi是饮食或非饮食途径的日平均摄入量(mg·kg−1·d−1);HQ为非致癌风险;CR为致癌风险,其它参数见详见表1. 对于非致癌物质,若HQ>1,则该物质表现出非致癌风险;对于致癌物质,若CR>10−6,则该物质表现出致癌风险,通常认为CR>10−4是不可接受的.
表 1 健康风险评估模型的参数Table 1. Parameter factors of the health risk assessment model参数Parameter 参数含义Meaning 单位Units 取值 Value 参考文献Reference 成人 Adults 儿童 Children IRS 土壤日均摄入量 mg·d−1 100 200 [17] IRF 居民日均摄取食物量 mg·d−1 710000 1/3×710000 [17] EF 暴露土壤频率 d·a−1 350 [17] ED 暴露持续时间 a 24 6 [17] BW 体重 kg 70 15 [17] AT 平均暴露时间 a 致癌风险:25550非致癌风险:365×ED [17] CF 转换系数 — 10−6 [17] SA 暴露皮肤的表面积 cm2 d−1 57000 28000 [17] AF 土壤-皮肤黏附系数 mg·cm−2 0.07 0.2 [17] ABS 从土壤中吸收的污染物的比例 — 0.1 [17] Ij 呼吸速率 m3·d−1 13.5 [17] PEF 微粒排放因子 m3·kg−1 1.36×109 [17] CFS 致癌率 (mg·kg−1·d−1)−1 BBP:0.0019 DEHP:0.014 [18] RfDi 非致癌物经某种途径摄入的日均推荐剂量 mg·kg−1·d−1 DMP:10 DEP:0.8DBP:0.1 BBP:0.2DEHP:0.02 DnOP:0.04 [18] BAF PAEs从土壤到食物的富集系数 — DMP:0.108 DEP:0.108DBP:0.108 BBP:0.166DEHP:0.166 DnOP:0.166 [18] 2. 结果与讨论 (Results and discussion)
2.1 洋河农业区PAEs总含量与单体污染特征
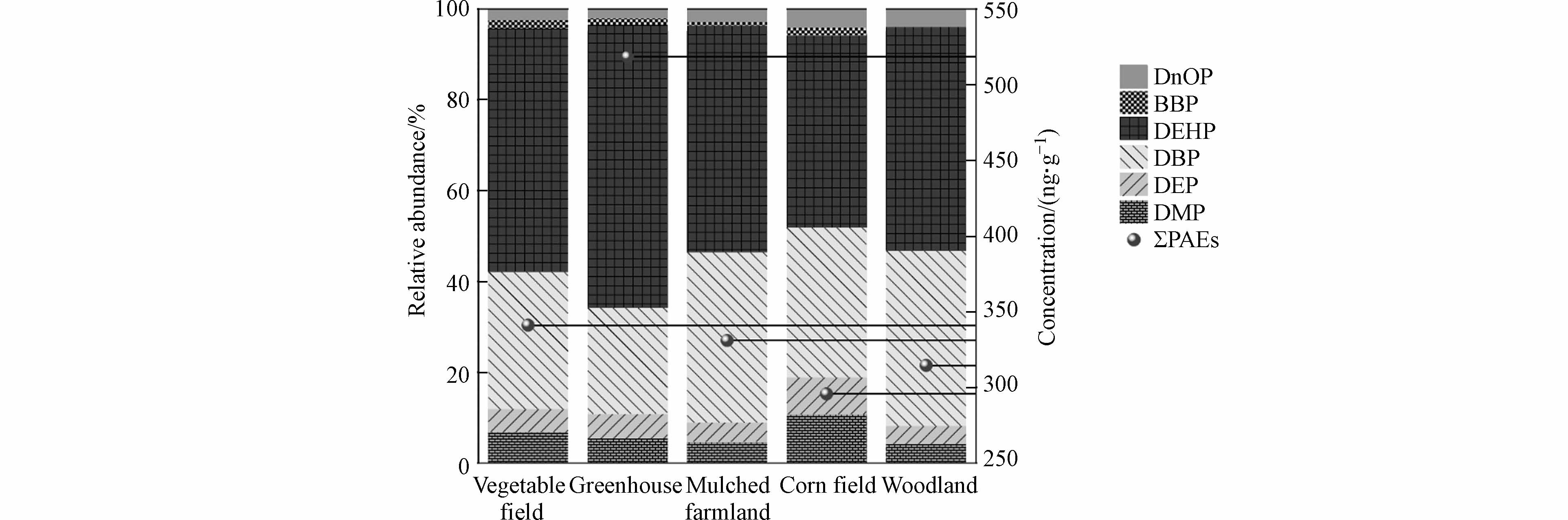
洋河流域农业土壤中PAEs各单体含量以及检出情况见表2. 6种PAEs单体在土壤中的平均含量顺序依次为DEHP>DBP>DMP>DEP>BBP>DnOP. 除DnOP外,其余单体检出率在80%以上,意味着这些物质在该地区被广泛使用. Σ6PAEs含量范围在183.63—780.50 ng·g−1,平均值和中位数分别为396.75 ng·g−1和365.87 ng·g−1. DEHP是土壤中最丰富的单体,含量范围在70.77—473.15 ng·g−1,平均值为219.98 ng·g−1,占Σ6PAEs的平均百分比为55.45%. 其次是DBP,占比为28.74%. DMP和DEP含量相当,占比分别为6.21%和5.60%. BBP和DnOP的占比相对较低,二者总占比仅为4.00%. 其中,DMP和DBP均超过了美国纽约州制定的土壤PAEs控制标准,超标率为32%和48%,最高超标分别达到3.1倍和3.5倍,值得关注和重视.
表 2 洋河流域土壤中PAEs含量、检出率与超标率Table 2. Concentration, detected ratio and excess ratio of soil PAEs in Yanghe River Basin化合物Compound DMP DEP DBP BBP DEHP DnOP Σ6PAEs 最小值/(ng·g−1) nd 7.30 46.87 nd 70.77 nd 183.63 最大值/(ng·g−1) 61.65 45.24 284.24 36.53 473.15 23.40 780.50 中值/(ng·g−1) 23.33 19.82 107.06 11.40 176.04 nd 365.87 平均值/(ng·g−1) 24.65 22.21 114.04 10.57 219.98 5.30 396.75 检出率/% 91.07 100 100 80.36 100 39.29 100 USEPA控制标准/(ng·g−1) 20 71 81 1215 4350 1200 — 超标率/% 32.00 0 48.00 0 0 0 — 注:“nd”表示未检出. Note: “nd” means no detect. 洋河农业区土壤中PAEs组成以DEHP和DBP为主,与西安城市[19]、雷州半岛[20]报道的情况类似. PAEs各单体含量差异,可能与单体性质、环境来源以及土壤的理化性质和环境条件有关[14]. DEHP和DBP由于分子量相对较大,水溶性较低,辛醇-水分配系数(lgKow)较大,在土壤中易于吸附富集,流动性较差,导致质量百分比偏高;而DMP、DEP等短链PAEs的水溶性相对较高,lgKow相对较小,相比于较长的烷基碳链的PAEs,更易被生物降解或通过挥发、淋溶和植物吸收等途径消失[21]. 另外,DEHP、DBP是农膜中使用最广泛的增塑剂,在各种有机肥料中也被大量检测到,因此在农业土壤中的应用范围更广.
如表3所示,洋河农业区PAEs污染程度与北京温室和泰安温室土壤基本相似,高于东北三江平原和黄河三角洲,但低于银川和天津菜地土壤. 不难看出土壤中PAEs污染水平有一定的区域差异性,分析这种差异可能归因于地区间不同的灌溉方式、土地利用方式和种植结构等[13]. 洋河流域是青岛市典型的灌溉农业区,利用洋河水源沿流域两岸农田自流灌溉. 关于灌溉水中的PAEs污染已有报道,特别是在地表河流和湖泊中[22]. 尽管地表水环境的PAEs含量低得多,但随着时间的推移,河水从灌溉源到农田的运输过程可能会导致PAEs在土壤中的积累. 与印度和英格兰相比,洋河农业区土壤中PAEs各单体污染水平明显偏高,这与我国的人口基数与塑料制品的需求量大密切相关.
表 3 不同地区不同土地利用类型中PAEs含量 (ng·g−1)Table 3. Comparison of concentration of PAEs in different land use types soils from different regions (ng·g−1)地点Location 土地类型Land type DMP DEP DBP BBP DEHP DnOP Σ6PAEs 参考文献Reference 北京 温室 8.0 20.0 440.0 4.0 380.0 2.0 850.0 [8] 杭州西湖 景区 432.8 41.0 519.9 2.1 1964.2 3.3 2963.3 [23] 天津 农田 3—88 3—81 7—189 nd—1790 39—2370 nd—647 91—2740 [13] 果园 3—32 3—30 20—138 nd—125 26—358 nd—728 53—1080 菜地 2—101 2—114 13—285 nd—385 28—4170 nd—9780 50—10400 黄河三角洲 乡村 1—5 nd—1 136—1039 nd 431—2449 nd—68 716—3251 [24] 公园 6—16 1—6 15—141 nd—1 43—801 0—16 70—923 咸阳 蔬菜基地 53.3 19.5 316.6 42.3 166.2 39.5 639.3 [14] 三江平原 蔬菜 12—34 20—46 22—209 nd—5317 34—218 nd—54 163—469 [25] 水稻 13—49 33—97 15—354 nd—72 77—583 nd—163 268—947 银川 蔬菜 89—3293 210—1381 139—2653 nd—1055 330—6017 nd—1713 1997—11659 [26] 泰安 温室 nd nd 110—166 19—22 199—564 20—27 350—767 [12] 印度 城市土壤 nd—8 9—12 8—36 20—35 16—30 nd—33 53—154 [27] 英格兰 城市黏土 0.1 0.9 8 0.6 76 14 92.6 [28] 青岛洋河 农业土壤 nd—61.7 7.3—45.2 46.9—284.2 nd—36.5 70.8—473.2 nd—23.4 183.6—780.5 本研究 注:“nd”表示未检出. Note: “nd” means no detect. 2.2 洋河农业区不同类型土壤中PAEs污染水平与组成特征
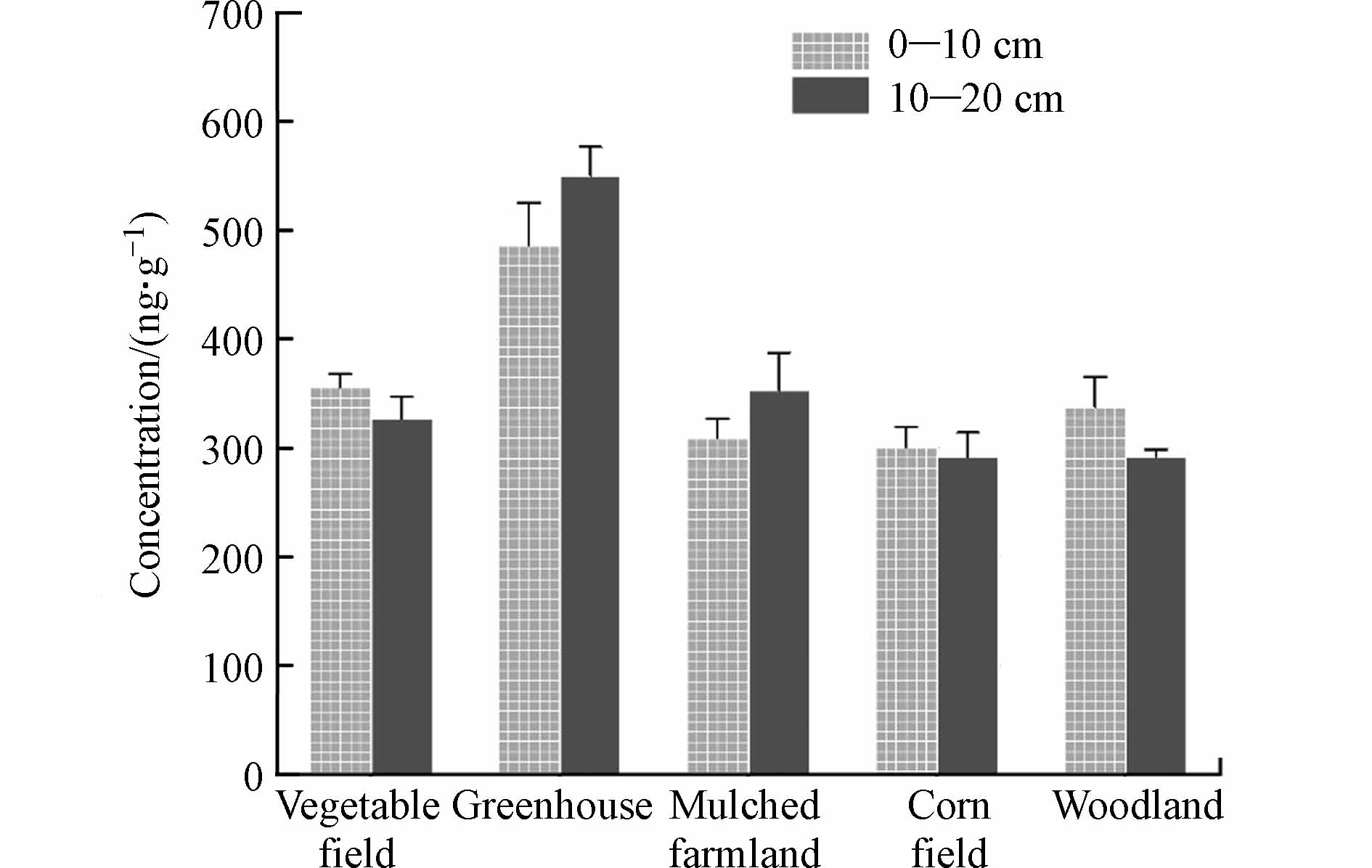
洋河农业区不同类型土壤中Σ6PAEs的含量情况见表4. Σ6PAEs的平均含量由高到低依次为:温室土壤(518.38 ng·g−1)>蔬菜地(341.13 ng·g−1)>覆膜耕地(331.02 ng·g−1)>林地(314.56 ng·g−1)>玉米地(295.74 ng·g−1). 整体上,不同类型土壤的Σ6PAEs含量差异不显著,不存在严重的局部偏差. 可能是该地区以农业生产为主,远离城市和工厂,工业活动和城市垃圾产生的PAEs有限.
表 4 洋河农业区不同土地类型土壤中PAEs的含量 (ng·g−1)Table 4. The PAEs concentration in soil of different types of land in Yanghe River Basin (ng·g−1)土地类型Land type Σ6PAEs 范围Range 平均值±标准差Mean±SD 中值Median 总数(n=56) 183.63—780.50 396.75±146.35 365.87 温室土壤(n=22) 191.51—780.50 518.38±155.71 467.12 覆膜耕地(n=8) 241.47—482.69 331.02±75.69 330.63 玉米地(n=12) 183.63—377.91 295.74±69.48 299.29 蔬菜地(n=8) 277.62—404.34 341.13±46.28 348.17 林地(n=6) 278.38—415.48 314.56±50.93 298.34 温室土壤中PAEs来源较为复杂. 一方面与棚膜的使用有关,另一方面,温室频繁地进行人为干预,如大水漫灌、化肥与农药杀虫剂大量施用等[29],这种集约化管理方式导致温室土壤中PAEs浓度显著高于其它农业土壤;地膜只在作物生长季节铺设,覆盖时间短,释放出的PAEs较少,且通过淋溶、下渗等途径迁移[18],因此覆膜耕地中残留的PAEs低于温室土壤;相比之下,蔬菜地由于不受地膜的阻挡或庇护,来自有机肥料、生活垃圾和道路交通灰尘的外部输入都可能是土壤中PAEs的重要来源;大气沉降或许是造成林地中PAE累积的原因:PAEs在土壤大气环境中的交换主要通过两个途径:一是大气中黏附有PAEs的气溶胶颗粒通过干沉降和湿沉降的方式进入土壤,二是土壤赋存态PAEs向大气环境中挥发[30],其中沉降为主要途径. 相对于普通露天农田而言,林地具有一定的阻挡作用,大气中的PAEs更容易在此处沉降[18];本研究中玉米种植土壤中PAEs含量最低,可能是因为采样时间为9月,临近玉米收获期,土壤耕作以及化肥和农药的施用频率降低.
评估土壤中PAEs污染情况,仅考虑其总浓度是不够的,还应考虑污染物单体的组成和浓度范围(图2). 尽管不同农业土壤中PAEs单体平均含量不尽相同,但贡献率较高的单体(DEHP、DBP)与研究区主要污染组成情况相一致. 其中,DEHP占Σ6PAEs百分比范围分别是42.42%—62.26%,顺序依次为温室土壤(62.26%)、蔬菜土壤(53.52%)、覆膜耕地(49.90%)、林地土壤(49.31%)、玉米土壤(42.42%);而DBP在不同土壤类型中的占比相差不大,占比范围在23.36%—38.63%.
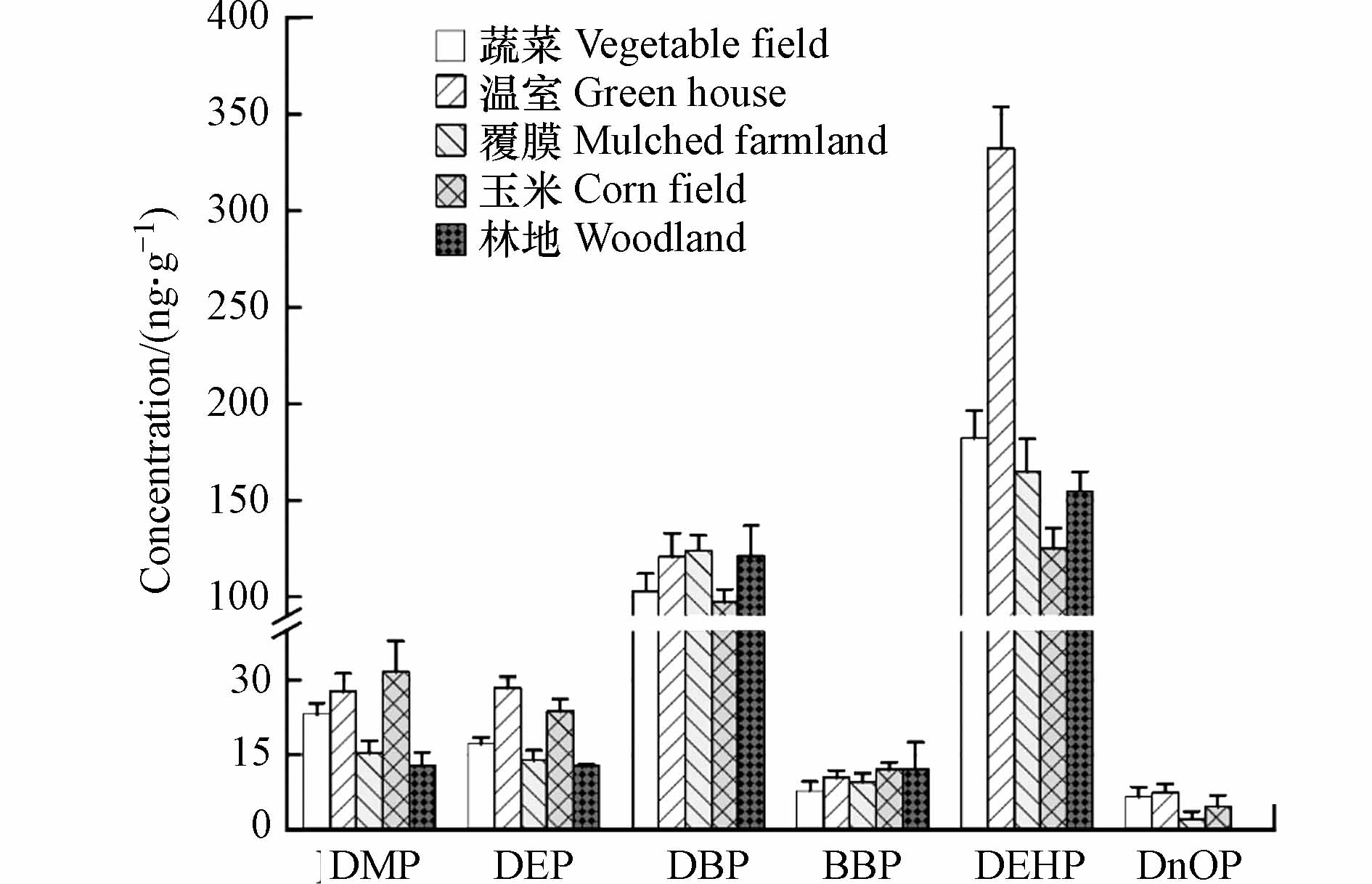
就单体检出含量来看(图3),DBP和DEHP在温室土壤中的平均浓度均高于蔬菜地、玉米地和林地,其余几种单体未见显著增高. 相反,玉米土壤中DMP和DEP的平均浓度较温室土壤还略高.
对于覆膜土壤而言,DBP的平均含量和占比高于3种露天土壤,这与DBP常作为增塑剂这一事实一致,但只对玉米土壤来说差异显著(P=0.019,<0.05). 这可以解释为,采样时间为9月,覆膜土壤经过了整个夏季的太阳辐射和雨季降水. 尽管DBP较高的溶解度和蒸汽压使其容易从塑料薄膜中渗出,但覆膜环境下适宜的温度和湿度条件可能会促进DBP的降解,从而打破土壤中PAEs输入和减少之间的平衡. Wu等[31]的研究支持了这一结果:随着温度从25 °C升高到30°C,土壤中DBP的降解速率迅速增加. Wang等[32]将聚乙烯农膜埋在土壤中4年,农膜中的PAEs浓度降低了79.2%—98.0%,然而农膜覆盖下的土壤与附近土壤背景之间几乎没有发现PAEs浓度的差异. Xie等[33]报道,DBP在28 °C时的半衰期为8.53 d,意味着农膜中释放的DBP在土壤中的保留时间较短. 事实上,覆膜土壤中其余单体含量(DEHP除外)均处于较低水平,说明PAEs确实容易在该环境下降解. 同样作为塑料制品中使用最广泛的增塑剂,覆膜土壤中的DEHP呈现出与DBP类似的规律,但平均浓度要高于DBP. DEHP的半衰期在18.5—41.4 d[32],是DBP的8—10倍,相比之下较难被微生物降解. 另外,DEHP是有机肥料中最常被检测到的物质,地膜的覆盖一定程度上阻碍其输入,因此DEHP浓度小于蔬菜土壤也就不足为奇了. 由此可见,地膜覆盖对土壤中PAEs的残留影响较小.
2.3 洋河流域农业区不同类型土壤中PAEs的垂直分布
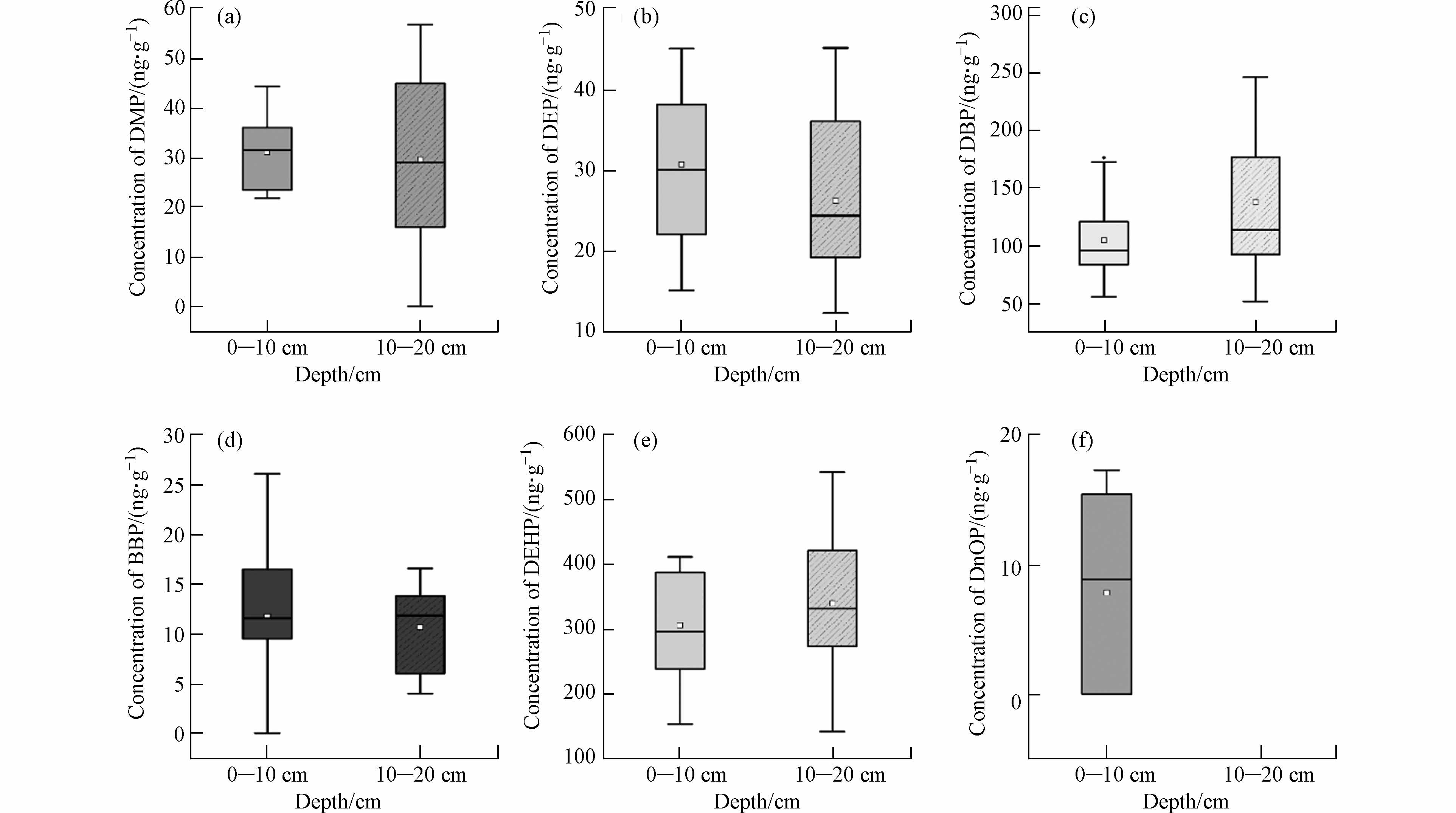
一些研究表明,随着土壤深度的增加,土壤中PAEs浓度逐渐降低[34]. 然而该规律仅在蔬菜地、玉米地和林地中得以体现(图4),原因可能是林地土壤质地紧实、偏砂质,PAEs更倾向于在地表积累而难以渗透到深层当中. 已经证实,植物根部的PAEs的含量显著高于土壤中,意味着植物可以通过根部从土壤中吸收和积累PAEs[35]. 因此对于玉米和蔬菜地而言,体现在10—20 cm土壤中PAEs的含量的低于0—10 cm. 这种趋势与温室和覆膜耕地明显不同,土壤的频繁翻转可以部分解释这种差异,特别是对于温室来说,频繁翻转可能会增加土壤中PAEs的挥发并促进好氧生物降解,从而降低PAEs在表层土壤中的浓度.
本研究土壤中(蔬菜地、玉米地和林地)呈现的变化趋势与江汉农田土壤不一致[36],这种差异可能归因于降雨对PAEs浸出的影响. 江汉平原处于水循环非常活跃的气候潮湿地区,通过土壤浸出的水会将PAEs沿土壤剖面输送到深处. 由于此处相对缺乏氧气和微生物质,PAEs的降解速率受限,因此土壤深层的PAEs含量较高. 在洋河流域,农业耕地通常每半年一犁,温室则更为频繁,这有助于PAEs在浅层和深层之间的垂直转移,可能是导致不同层间PAEs含量差异不够显著的原因. 总体而言,不同研究中PAEs垂直分布可能与许多因素有关,包括土壤耕作、采样深度、降水以及生物和机械干扰[10].
另一个值得关注的问题是PAEs单体随深度的变化情况. 以温室为例,DnOP在深层土壤中均没有被检出,其余5种单体均存在下渗的情况,但分布规律不尽相同(图5). DMP在各层间含量相当,由于水溶性和易于降解,因此在土壤各层中污染程度不高;DBP有与相似的物理性质和化学结构,其分布规律与DMP类似. DBP溶解度大、易于下渗,温室土壤灌溉频率较高,因此更容易向土壤深处迁移. DEHP作为最主要污染物,分布情况与Σ6PAEs相同,主要集中在深层,但各层的残留也较高. BBP土壤中污染程度小,在深层土壤中很少检出.
2.4 PAEs含量与土壤理化性质的相关性
为了进一步了解洋河农业区PAEs的各种潜在来源以及典型理化性质对土壤中PAEs积累之间的关系,本研究计算了PAEs单体残留水平与土壤理化性质之间的Spearman相关系数,如图6所示. 结果表明,土壤中发现的主要单体(DEP、DBP和DEHP)的浓度彼此之间有明显的相关性(P<0.05),并且这3种单体与Σ6PAEs密切相关(r=0.67、0.62和0.93;P<0.01). 这些相关性可能表明各种单体在土壤中可能有类似的输入途径和命运.
TOC、TN和pH是影响包括PAEs在内的疏水性有机污染物(HOCs)行为的重要因素. 本研究区域土壤中TOC、TP和pH值范围为0.130%—1.348%、0.001‰—0.086%和4.92—7.74,平均值为0.423%、0.031%和6.47. 其中,TOC值远低于北京(0.97%)[37]、寿光(1.14%)[38]等地区. 与其它HOCs一样,PAEs亲油性强、易与有机物结合,这一物理化学特性使得PAEs很大程度上会被土壤有机质吸附[39],因此研究区土壤中较低的TOC含量可能是导致土壤捕获PAEs较低的原因. 然而PAEs和TOC之间没有相关性,可能是环境中存在连续的HOCs来源,导致HOCs与TOC之间不相关[40]. 在洋河农业区,农药、化肥与农用塑料被频繁使用或更换,这些含有丰富PAEs的新鲜材料被连续引入土壤中,可能会打破PAEs和TOC之间的平衡. 在山东半岛的温室土壤中也发现了类似的现象[41]. 另外,本研究中也没有发现PAEs与土壤pH和TN值之间的相关性.
2.5 洋河农业区土壤中PAEs的来源解析
污染物的环境来源诊断对于风险管理和环境修复具有重要意义. 本研究中,主成分分析和聚类分析被用于识别PAEs的环境来源. 考虑到本研究区域可用于林地、覆膜和蔬菜的样本量相对有限,因此将所有土壤样本组合在一起进行分析. 土壤中PAEs主成分分析(KMO值为0.756,Bartlett's球形检验显著)结果见表5,使用kaiser进行标准化正交旋转,提取了4个主成分。
表 5 洋河农业区土壤PAEs主成分分析结果Table 5. Results of principal component analysis of PAEs in soils of Yanghe agricultural area化合物PAEs 主成分因子Principal component factors PC1 PC2 PC3 PC4 DMP 0.13 0.93 0.12 0.09 DEP 0.34 0.55 0.57 0.13 DBP 0.14 0.12 0.95 0.08 BBP 0.05 0.10 0.10 0.99 DEHP 0.68 0.33 0.23 -0.03 DnOP 0.90 0.03 0.08 0.09 方差贡献率/% 43.75 15.82 12.96 11.19 方差累计贡献率/% 43.75 59.57 72.52 83.71 第一主成分(PC1)方差贡献率为43.75%,DEHP和DnOP载荷较高,单体之间呈显著正相关(r=0.43,P<0.05),说明它们可能有相似的来源. 研究表明,DEHP和DnOP主要用作增塑剂[1]. 其中,DEHP是应用最为广泛的PAEs类增塑剂,能添加到于大多数塑料中,如PVC. DnOP也应用于乙烯地板的生成,油墨、皮革等橡胶制品中[42].
第二主成分(PC2)方差贡献率为15.82%,DMP的载荷最高. 主要作为农药和杀虫剂中的溶剂,化妆品和个人护理品中也有所检出[43].
第三主成分(PC3)方差贡献率为12.96%,由DBP与部分DEP构成,呈现显著相关性(r=0.54,P<0.05). 有报道称化肥中含有DBP和DEP[43]. 其次DBP也是常用的PAEs类增塑剂之一.
第四主成分(PC4)方差贡献率为11.19%,BBP载荷最高. BBP多来源于增塑剂,建筑和装饰材料的挥发,以及含有PAEs颗粒的大气沉积[42].
聚类分析(图7)将土壤中的PAEs分为4类:DEP和DBP为类1,DEHP和DnOP为类2,DMP、BBP分别为类3和类4,与主成分分析结果一致. 同时,类1、类2和类3在高更层次又形成了的一个新的集群,这反映出它们可能还有另一个共同的环境来源.
通常认为,PAEs的支链长度与来源相关[44]. 农业生态系统中,短链PAEs,如DMP和DEP,通常来源于农药、污灌和有机肥;而长链PAEs,如DBP、DEHP、BBP和DnOP,在聚合物工业中被广泛用作增塑剂,约80%的PAEs用于此目的,在聚体中的含量从10%—60%不等[1]. 本研究区的PAEs主要以DBP和DEHP为主,这与我国主要使用它们为增塑剂的事实一致,但主成分分析和聚类分析结果表明这两种单体并不属于一类,说明它们可能具有复合来源. 实地考察发现,洋河农业区土壤中PAEs的来源具有一定的复杂性和广泛性. 一方面,这两种单体广泛存在于各个环境介质(水、土壤和大气)和农业投入品(塑料增塑剂、有机肥和污灌)中[45]. 另一方面,覆膜下适宜的湿度和温度促使农膜中释放的PAEs迅速减少,特别是对DBP来说. 相比之下BBP和DnOP来源较为单一,主要作为增塑剂随塑料制品进入土壤,这也就解释了为什么BBP和DnOP在洋河农业区不同类型土壤中显示出较强的特异性,体现在3种露天农田土壤中的检出率和含量都很低.
2.6 洋河农业区土壤中PAEs的健康风险评估
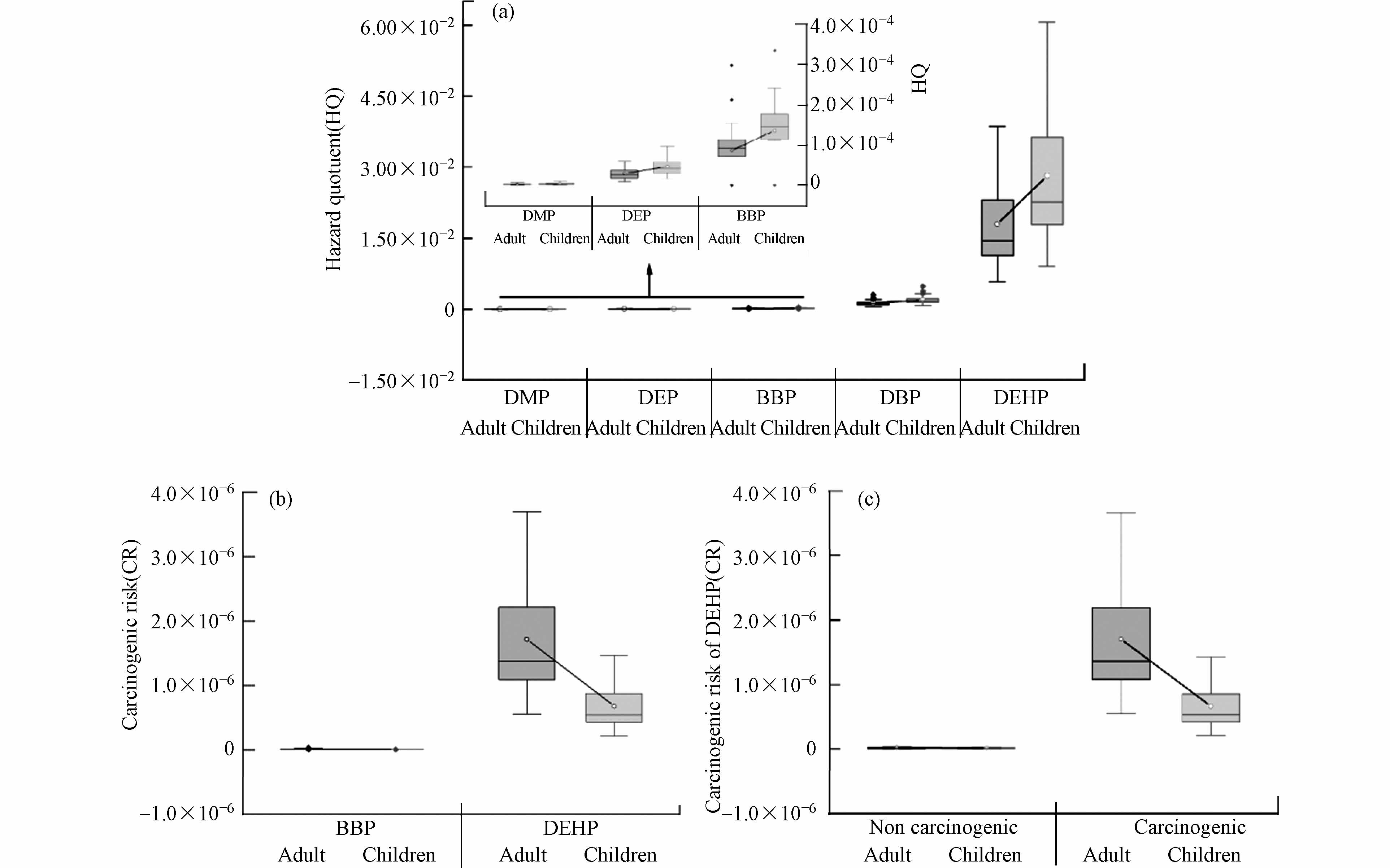
根据1.6中的健康风险模型,计算出不同暴露途径下成人和儿童对PAEs的日均摄入量(表6). 由于DnOP检出率较低,因此不进行计算. 从各单体对日均摄入量的贡献来看,无论是对于成人还是儿童,DEHP所占比例最大,约为65.0%. 从各种暴露途径来看,饮食途径的日均摄入量均显著高于非饮食途径,其对日均摄入量的贡献率分别为成人的98.5%和儿童的86.8%. 非饮食途径中,成人的PAEs的日均摄入量顺序为呼吸摄入>皮肤接触>土壤摄入,儿童则为皮肤接触>土壤摄入>呼吸摄入. 值得注意的是,尽管从事农业生产的主要是成年人,但成年人对PAEs的总日均摄入量明显低于儿童,这表明儿童可能更容易从土壤环境中摄入PAEs.
表 6 不同暴露途径下成人和儿童对PAEs的日均摄入量(mg·kg−1·d−1)Table 6. The ADD values of PAEs for adults and children via different exposure pathway(mg·kg−1·d−1)化合物PAEs 类别Type 饮食途径Dietary routes 非饮食途径Non-Dietary routes 饮食摄入Intake 土壤摄入Ingest 皮肤接触Dermal 呼吸摄入Inhale DMP 成人 2.59×10−5 3.37×10−8 1.35×10−7 2.35×10−7 儿童 4.02×10−5 3.16×10−7 8.83×10−7 2.35×10−7 DEP 成人 2.33×10−5 3.04×10−8 1.21×10−7 2.11×10−7 儿童 3.62×10−5 2.84×10−7 7.95×10−7 2.11×10−7 DBP 成人 1.20×10−4 1.56×10−7 6.23×10−7 1.09×10−6 儿童 1.86×10−4 1.46×10−6 4.08×10−6 1.09×10−6 BBP 成人 1.71×10−5 1.45×10−8 5.78×10−8 1.00×10−7 儿童 2.65×10−5 1.35×10−7 3.78×10−7 1.00×10-7 DEHP 成人 3.55×10−4 3.01×10−7 1.20×10−6 2.09×10−6 儿童 5.51×10−4 2.81×10−6 7.88×10−5 2.09×10−6 图8a显示,各单体的非致癌风险值从大到小依次为DEHP(1.79×10−2和2.82×10−2)、DBP(1.22×10−3和1.93×10−3)、BBP(8.62×10−5和1.36×10−4)、DEP(2.96×10−5和4.69×10−5)和DMP(2.60×10−6和4.11×10−6),不同PAEs均未对人体健康产生非致癌风险(HQ<1). 尽管如此,DEHP和DBP的危害熵值显著高于DMP、DEP和BBP,表明这两种污染物的非致癌风险相对较高. 儿童表现出比成人更高的非致癌风险,这可以归因于由于身体不成熟和新陈代谢弱而对毒性的易感性[46]. 图8b可以看出,BBP的致癌风险非常低,但DEHP对成人和儿童致癌风险范围在5.53×10−7—3.70×10−6和2.19×10−7—1.46×10−6,分别有83.93%和17.86%的采样点超过USEPA推荐值,呈现出一定的致癌风险,但仍在可接受的范围内(10−6<CR<10−4). 与非致癌风险不同,成年人表现出更高的致癌风险,可能因为成人对污染物的摄入是持续且长期的,导致更高的致癌性[46]. 考虑到儿童本身抵抗力低,因此对于儿童的潜在致癌风险不容忽视.
由于BBP的风险可忽略不计,因此选择DEHP进一步评估分别在非饮食和饮食途径下的致癌风险. 图8c可以看出,无论是成人还是儿童,致癌风险都是由饮食途径引发的,致癌风险范围在6.65×10−7—3.66×10−6,平均值为(1.18±0.82)×10−6. 有研究表明,土壤中的DEHP能够通过食物链的富集作用对人体健康构成严重的威胁,一方面因为DEHP在土壤中的浓度较大,还受其生物积累因子(BAF)及每日摄入受污染食物的最大参考剂量(RfDo)的影响[45],因此有必要对其进行进一步研究,以充分了解土壤环境中PAEs的健康风险和机制.
3. 结论(Conclusion)
通过对青岛洋河流域典型农业土壤中6种PAEs的浓度和分布的研究,发现各单体均有不同程度检出,土壤受到一定程度的污染.
(1)土壤中Σ6PAEs含量范围在183.63—780.50 ng·g−1之间,平均值为432.4 ng·g−1. DBP与DEHP是研究区土壤中PAEs的主要组成部分. DMP和DBP含量超过美国纽约州土壤PAEs控制标准.
(2)不同农业土壤中Σ6PAEs平均值大小顺序依次为:温室土壤>蔬菜地>覆膜土壤>玉米地>林地. 蔬菜地、玉米地和林地表层中6种PAEs高于深层,温室和覆膜耕地则相反. PAEs分布多与农业活动有关,并受土壤微生物降解的影响.
(3)土壤中主要单体(DEP、DBP和DEHP)的浓度间有明显的相关性(P<0.05). 统计分析表明,农用塑料、施肥和农药杀虫剂是洋河农业区土壤中PAEs的主要来源.
(4)各单体对成人和儿童产生的非致癌风险均未超过美国EPA推荐的非致癌水平(HQ<1),但DEHP在饮食暴露途径下对居民致癌风险超过了阈值水平(CR>10−6). 今后应采取相应措施,更加关注PAEs在土壤中的污染状况以及对当地居民健康的潜在影响.
-
表 1 反应器连续运行工况
Table 1. Continuous operating conditions of oxygen-limited aeration reactor
反应器 阶段 运行时间/d 进水流量/(L∙h−1) NH4+-N/(mg∙L−1) NO2−-N/(mg∙L−1) NO3−-N/(mg∙L−1) PO43-−P/(mg∙L−1) 温度/℃ pH 曝气量/(mL∙min−1) DO/(mg∙L−1) R1 抑制期 1~73 0.22 150 200 0~10 10 20±5 7.3~7.5 0 0.1~0.4 R2 过渡期 74~91 — 125 50 0~10 10 25 7.8~8.0 100 0.4~0.7 好氧期 92~130 — 200 0 0~10 10 25 7.5~8.0 200 0.7~1.0 -
[1] 张铃敏, 常青龙, 史勤, 等. CANON工艺短程硝化恢复调控及微生物种群结构变化[J]. 中国环境科学, 2019, 39(6): 2354-2360. doi: 10.3969/j.issn.1000-6923.2019.06.015 [2] ZUO L S, YAO H, LI H Y, et al. Modeling of completely autotrophic nitrogen removal process with salt and glycine betaine addition[J]. Chemosphere, 2020, 264(2): 1-8. [3] STROUS M, KUENEN G J, JETTEN M S M. Key physiology of anaerobic ammonium oxidation[J]. Applied and Environmental Microbiology, 1999, 65(7): 3248-3250. doi: 10.1128/AEM.65.7.3248-3250.1999 [4] 聂铭, 李振轮. 水体中亚硝酸盐积累的生物过程及影响因素研究进展[J]. 生物工程学报, 2020, 36(8): 1493-1503. [5] 张姚, 韩海成, 王伟刚, 等. 溶解氧对CANON颗粒污泥自养脱氮性能的影响[J]. 中国环境科学, 2017, 37(12): 4501-4510. doi: 10.3969/j.issn.1000-6923.2017.12.012 [6] HAN M, VLAEMINCK S E, AL-OMARI A, et al. Uncoupling the solids retention times of flocs and granules in mainstream deammonification: A screen as effective out-selection tool for nitrite oxidizing bacteria[J]. Bioresource Technology, 2016, 221: 195-204. doi: 10.1016/j.biortech.2016.08.115 [7] 李冬, 崔少明, 梁瑜海, 等. 溶解氧对序批式全程自养脱氮工艺运行的影响[J]. 中国环境科学, 2014, 34(5): 1131-1138. [8] 王会芳, 付昆明, 左早荣, 等. 水力停留时间和溶解氧对陶粒CANON反应器的影响[J]. 环境科学. 2015(11): 4161-4167. [9] JOSS A, DERLON N, CYPRIEN C, et al. Combined nitritation-anammox: Advances in understanding process stability[J]. Environmental Science & Technology, 2011, 45(22): 9735. [10] GRAAF A V D, BRUIJN P D, ROBERTSON L A, et al. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor[J]. Microbiology. 1996, 142(8): 2187-2196. [11] 付昆明, 周厚田, 苏雪莹, 等. 生物膜短程硝化系统的恢复及其转化CANON工艺的过程[J]. 环境科学, 2017, 38(4): 1536-1543. [12] 刘成良, 李天煜, 刘可慧, 等. 氨氮废水的厌氧氨氧化生物脱氮研究[J]. 生态环境学报, 2010, 19(9): 2172-2176. doi: 10.3969/j.issn.1674-5906.2010.09.026 [13] ZEKKER I, RIKMANN E, TENNO T, et al. Modification of nitrifying biofilm into nitritating one by combination of increased free ammonia concentrations, lowered HRT and dissolved oxygen concentration[J]. Journal of Environmental Sciences, 2011, 23(7): 1113-1121. doi: 10.1016/S1001-0742(10)60523-2 [14] 赵文钊, 裴浩, 王超, 等. 校园污水脱氮除磷处理系统的运行策略及其对污泥性能的影响[J]. 环境工程学报, 2020, 14(11): 3053-3062. doi: 10.12030/j.cjee.201912128 [15] 李炳荣, 曹特特, 王林, 等. 低氧条件下A2/O工艺对城市污水脱氮处理的中试研究[J]. 中国环境科学, 2019, 39(1): 134-140. doi: 10.3969/j.issn.1000-6923.2019.01.014 [16] JETTEN M S M, NIFTRIK L V, STROUS M, et al. Biochemistry and molecular biology of anammox bacteria[J]. Critical Reviews in Biochemistry and Molecular Biology. 2009, 44(2/3): 65-84. [17] 毛文龙, 杨瑞丽, 王晓君, 等. 由anammox转为CANON工艺的调控策略及微生物响应特性[J]. 环境工程学报, 2021, 15(7): 2488-2501. doi: 10.12030/j.cjee.202103122 [18] 冯平, 周少奇. 常温下厌氧氨氧化生物膜反应器的启动研究[J]. 环境科学与技术, 2010, 33(6): 19-22,34. [19] VAN DER STAR W R L, MICLEA A I, VAN DONGEN U G J M, et al. The membrane bioreactor: a novel tool to grow anammox bacteria as free cells[J]. Biotechnology and Bioengineering, 2008, 101(2): 286-294. doi: 10.1002/bit.21891 [20] LU L, WANG B J, ZHANG Y, et al. Identification and nitrogen removal characteristics of Thauera sp. FDN-01 and application in sequencing batch biofilm reactor[J]. Science of the Total Environment, 2019, 690: 61-69. doi: 10.1016/j.scitotenv.2019.06.453 [21] 陈均利, 彭英湘, 刘锋, 等. 异养硝化-好氧反硝化菌脱氮特性研究进展[J]. 环境科学与技术, 2020, 43(5): 41-48. [22] SU J F, YANG S, HUANG T L, LI M, et al. Enhancement of the denitrification in low C/N condition and its mechanism by a novel isolated Comamonas sp. YSF15[J]. Environmental pollution, 2020, 256(1): 1-7. [23] 付昆明, 付巢, 李慧, 等. 主流厌氧氨氧化工艺的运行优化及其微生物的群落变迁[J]. 环境科学, 2018, 39(12): 5596-5604. [24] 付昆明, 杨宗玥, 廖敏辉, 等. 中试MBBR反应器启动CANON工艺及其短程硝化[J]. 环境科学. 2020, 41(3): 1393-1400. [25] HANAKI K, WANTAWIN C, OHAGKI S. Nitrification at low-levels of dissolved oxygen with and without organic loading in a suspended growth reactor[J]. Water Research, 1990, 24(3): 297-302. doi: 10.1016/0043-1354(90)90004-P [26] 杨庆, 杨玉兵, 杨忠启, 等. 溶解氧对短程硝化稳定性及功能菌群的影响[J]. 中国环境科学, 2018, 38(9): 3328-3334. doi: 10.3969/j.issn.1000-6923.2018.09.016 [27] USHIKI N, JINNO M, FUJITANI H, et al. Nitrite oxidation kinetics of two Nitrospira strains: The quest for competition and ecological niche differentiation[J]. Journal of Bioscience and Bioengineering. 2017, 123(5): 581-589. [28] 张亮, 张树军, 彭永臻. 污水处理中游离氨对硝化作用抑制影响研究[J]. 哈尔滨工业大学学报, 2012, 44(2): 75-79. doi: 10.11918/j.issn.0367-6234.2012.02.016 [29] CAO Y S, VAN LOOSDRECHT MARK C M, DAIGGER G T. Mainstream partial nitritation-anammox in municipal wastewater treatment: Status, bottlenecks, and further studies[J]. Applied Microbiology and Biotechnology, 2017, 101(4): 1365-1383. doi: 10.1007/s00253-016-8058-7 [30] ANTHONISEN A C, LOEHR R C, PRAKASAM T B, et al. Inhibition of nitrification by ammonia and nitrous acid[J]. Journal Water Pollution Control Federation, 1976, 48(5): 835-852. [31] LOTTI T, KLEEREBEZEM R, ABELLEIRA-PEREIRA J M, et al. Faster through training: The anammox case[J]. Water Research, 2015, 81(Sep.15): 261-268. [32] 孙延芳, 韩晓宇, 张树军, 等. 颗粒+絮体污泥CANON工艺的启动与SRT影响研究[J]. 环境科学, 2017, 38(2): 672-678. -




 下载:
下载: