-
可挥发性有机化合物(volatile organic compounds, VOCs)是大气污染物中一大类[1-3]。浓度较高的VOCs气体会刺激人的眼睛、鼻子或咽喉等,导致干咳头晕、恶心疲劳等症状。长期生活在受VOCs污染的环境中,人体的神经系统会被损害,并诱发癌症,故VOCs的治理刻不容缓[4-6]。
传统VOCs处理技术主要有燃烧法、催化氧化法、吸收吸附法等。其中,燃烧法的操作较为简单,但因危险性较高,故对安全防护的要求较高;催化氧化法不需要额外试剂,且产生污染物较少,但同时存在催化剂稳定性和寿命等限制[7-8];吸收法可将VOCs回收再利用,但需根据待处理VOCs种类使用特定吸收剂,普适性较差;吸附法常用活性炭作为吸附剂,净化率高,但活性炭使用寿命很短,需频繁更换。
低温等离子体(non-thermal plasma, NTP)技术是一种新型VOCs处理技术,相较于传统VOCs处理技术,具有适用性广、响应快速等特点,因而受到广泛关注[9-11]。在众多产生NTP的放电形式中,介质阻挡放电(dielectric barrier discharge, DBD)因其结构简单、可通过改变放电参数调控等离子体能量密度,且能处理较大流量气体等优势而被广泛研究。王保伟等[12]通过研究放电间距对单介质阻挡放电(single dielectric barrier discharge, SDBD) 等离子体降解甲苯的影响,发现随放电间距的增大,甲苯转化率和CO2选择性呈先增后降趋势。ZHAO等[13]使用双介质阻挡放电(double dielectric barrier discharge, DDBD)等离子体降解多种芳烃、烷烃、酮和酯类VOCs,发现电离能是影响所有VOCs降解效率的重要参数,电离能越大,降解效率越低。相较于SDBD放电腔,DDBD放电腔可很好地保护放电电极不受工作气体污染。
为进一步优化NTP技术,提升VOCs转化率,并降低NTP降解VOCs过程中产生的臭氧与有机副产物产量,催化剂协同技术被越来越多应用于NTP降解VOCs的体系中[10-11, 14-17]。在众多研究中,对催化剂性能的表征大多使用单种VOCs进行。然而,实际情况下待处理的VOCs组分复杂,催化剂在多组分VOCs的处理中的表现还鲜有报道。
本研究拟使用双介质阻挡放电(DDBD)反应器产生低温等离子体,以甲苯、丙酮及乙酸乙酯的混合气体作为待降解模拟VOCs混合废气[18-19],并制备常用于协同NTP降解VOCs的Mn2O3/γ-Al2O3催化剂,以研究NTP降解复杂成分VOCs的特性,以及催化剂对NTP降解混合VOCs的影响,以期为NTP降解VOCs的实际应用提供参考。
-
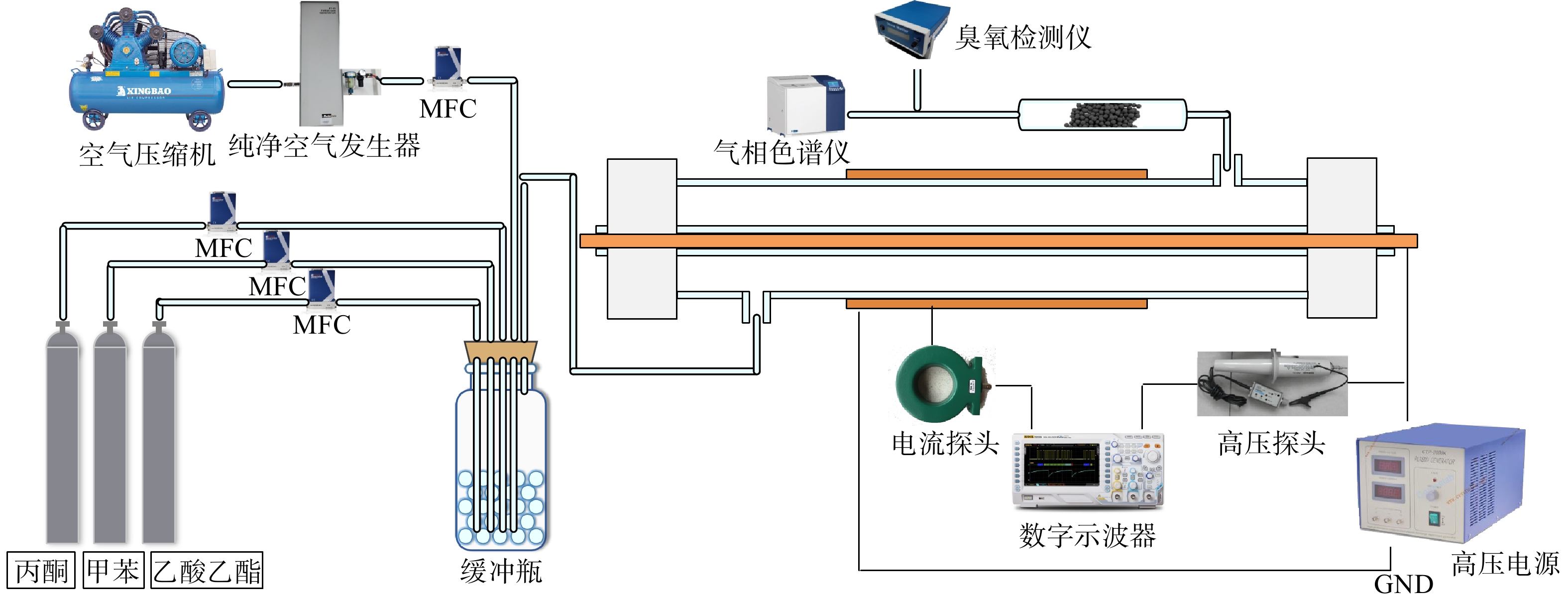
实验装置及流程图如图1所示。模拟混合VOCs废气由高浓度丙酮、甲苯及乙酸乙酯标气经稀释得到。稀释标气所用气体为经过纯净空气发生器干燥后的压缩空气。使用4个质量流量控制器(mass flow controller, MFC)分别控制丙酮、甲苯、乙酸乙酯及压缩空气的流量,以得到实验所需的各VOCs组分的初始浓度。模拟混合VOCs经缓冲瓶混合后通入DDBD反应器降解,模拟VOCs的流速固定在1 L ∙ min−1。在VOCs单独降解实验中,甲苯、丙酮及乙酸乙酯的初始体积分数均为(33±2)×10−6。在混合VOCs降解实验中,甲苯、丙酮及乙酸乙酯的初始体积分数也均为(33±2)×10−6。
DDBD反应器的2层介质分别为1根外径为20 mm,内径为17 mm的石英管(外管),以及1根外径为8 mm、内径为6 mm的石英管(内管)。内管中放置1根直径6 mm的铜棒作为高压电极,外管缠绕宽度为10 cm的铝箔作为接地电极。模拟混合VOCs经DDBD反应器进气口进入反应器内进行低温等离子体降解。经初步降解后的废气由反应器出气口进入催化剂反应管进行进一步反应。该催化剂反应管为内径5 mm、长度30 cm的石英管。
降解前后的VOCs、CO和CO2体积分数均使用气相色谱仪(GC2060ⅢA,上海锐敏仪器有限公司)在线测定。其中,VOCs的体积分数使用配置有HT-5型毛细管柱(柱长30 m,内径0.32 mm)的火焰离子化检测器(flame ionization detector, FID)检测;CO和CO2的体积分数使用配有甲烷化转化炉的FID检测器检测。气相色谱仪的检测条件设定为:炉温60 ℃,检测器温度140 ℃,进样器温度120 ℃,甲烷转换炉320 ℃。反应过程中生成的臭氧体积分数使用臭氧检测仪(GT-2000-k3, Korno)测定。
-
将一定量的Mn(NO3)2(AR,国药集团化学试剂有限公司)与2 g γ-Al2O3(球形,国药集团化学试剂有限公司)分散在含有分散剂聚乙烯吡咯烷酮(质量分数2%)(AR,国药集团化学试剂有限公司)、乙醇(质量分数12%)(AR,国药集团化学试剂有限公司)和去离子水的混合溶液中。其中,Mn(NO3)2的量取决于Mn元素与γ-Al2O3的质量比。将混合物超声分散1 h后转移进容积为100 mL的聚四氟乙烯瓶中,在140 ℃条件下放置6 h[20-21]。混合物冷却至室温后,用去离子水洗涤3次,并在60 ℃下干燥,最后在马弗炉中以500 ℃煅烧产物6 h以获得催化剂。
-
VOCs废气的降解效果通常使用降解率与碳平衡进行表征。其中,VOCs的降解率(degradation rate, DR)由式(1)计算得到[22]。
式中:
cin 与cout 分别为降解前后各VOCs组分的体积分数,10−6。VOCs降解后的碳平衡(carbon balance, CB)可通过式(2)计算得到。
式中:
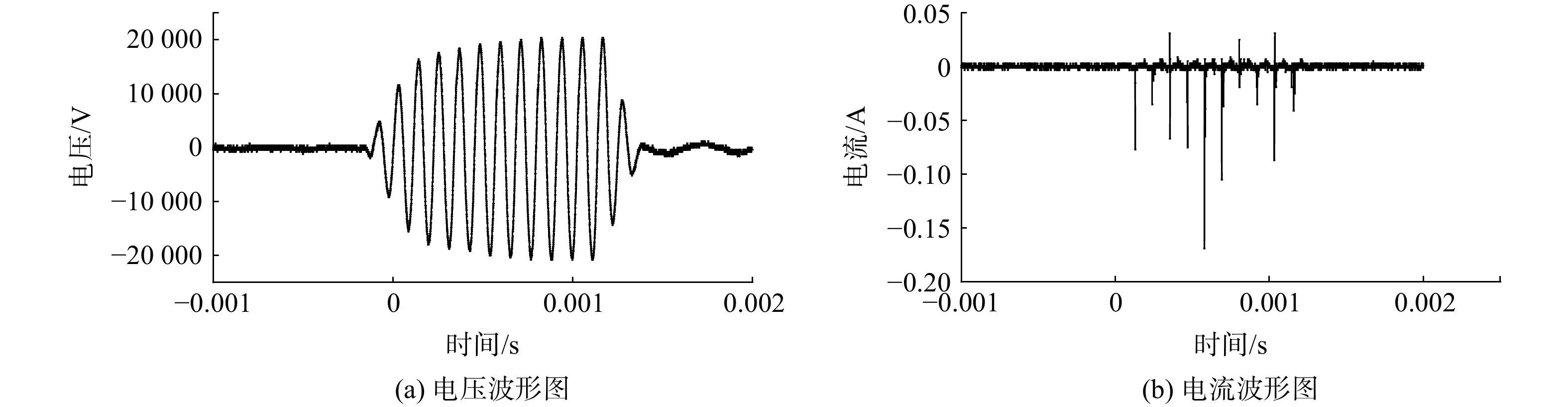
nCO 与nCO2 分别为VOCs降解产生的CO与CO2的体积分数,10−6;nT 、nE 以及nA 分别为被降解的甲苯、乙酸乙酯及丙酮的体积分数,10−6;数字7、4、3分别为甲苯、乙酸乙酯及丙酮分子中所含碳原子数。DDBD放电腔通过高压电源(CTP-2000K,南京苏曼电子有限公司)驱动放电,电源频率为10 kHz;放电腔两端的电压和电流分别通过高压探头( P6015A, Tektronix)及电流探头(5315, ETA)检测,并使用数字示波器记录(MDO3032, Tektronix)记录其放电波形。本课题组前期研究发现,调制脉冲电源可改善DDBD等离子体降解VOCs的能量效率。因此,在本实验中,电源通过一个矩形脉冲来调制一个中心频率为10 kHz 的正弦波形,并将高压电源的占空比和调制频率固定为20%与150 Hz。调制后的DDBD放电典型电流电压波形如图2所示。
反应器放电功率P可通过式(3)计算得到[23]。
式中:T为脉冲电源的脉冲宽度,s;f为调制脉冲频率,Hz; U(t)为高压探头测得的放电电压,V;I(t)为电流探针测得的放电电流,A。
进而可通过式(4)计算得到低温等离子体降解VOCs过程中的特定输入能量(specific input energy,SIE)。SIE是低温等离子体降解VOCs效果评价的重要参数之一[24]。
式中:Q为模拟VOCs废气的流速,L ∙ min−1。
-
催化剂的元素含量通过Prodigy ICP装置(利曼,美国)上的电感耦合等离子体(inductively coupled plasma, ICP)光电发射光谱进行测量。氮气吸附-脱附等温线在ASAP-2460分析仪上获得的。使用传统brunauer-emmett-teller(BET)和barrett-joyner-halenda(BJH)方程中的吸附数据确定催化剂的比表面积、孔径分布和孔体积;使用DD Max-2550PC型18 kW转靶X射线衍射仪(里加库,日本)记录催化剂粉末X射线衍射(X-ray diffraction, XRD)图;催化剂的X射线光电子能谱(X-ray photoelectron spectroscopy, XPS)由Thermo Escalab 250Xi型X射线光电子能谱仪(ThermoFisher,美国)在Al-K(1486.6 eV,150 W)辐射下获得;通过扫描电镜(scanning electron microscope, SEM)(JEOL 7800 F,日本)研究催化剂的形态。使用高分辨率透射电镜(high resolution transmission electron microscope, HR-TEM)(FEI Tecnai G2F30,美国)测定了催化剂的结构和元素图。
-
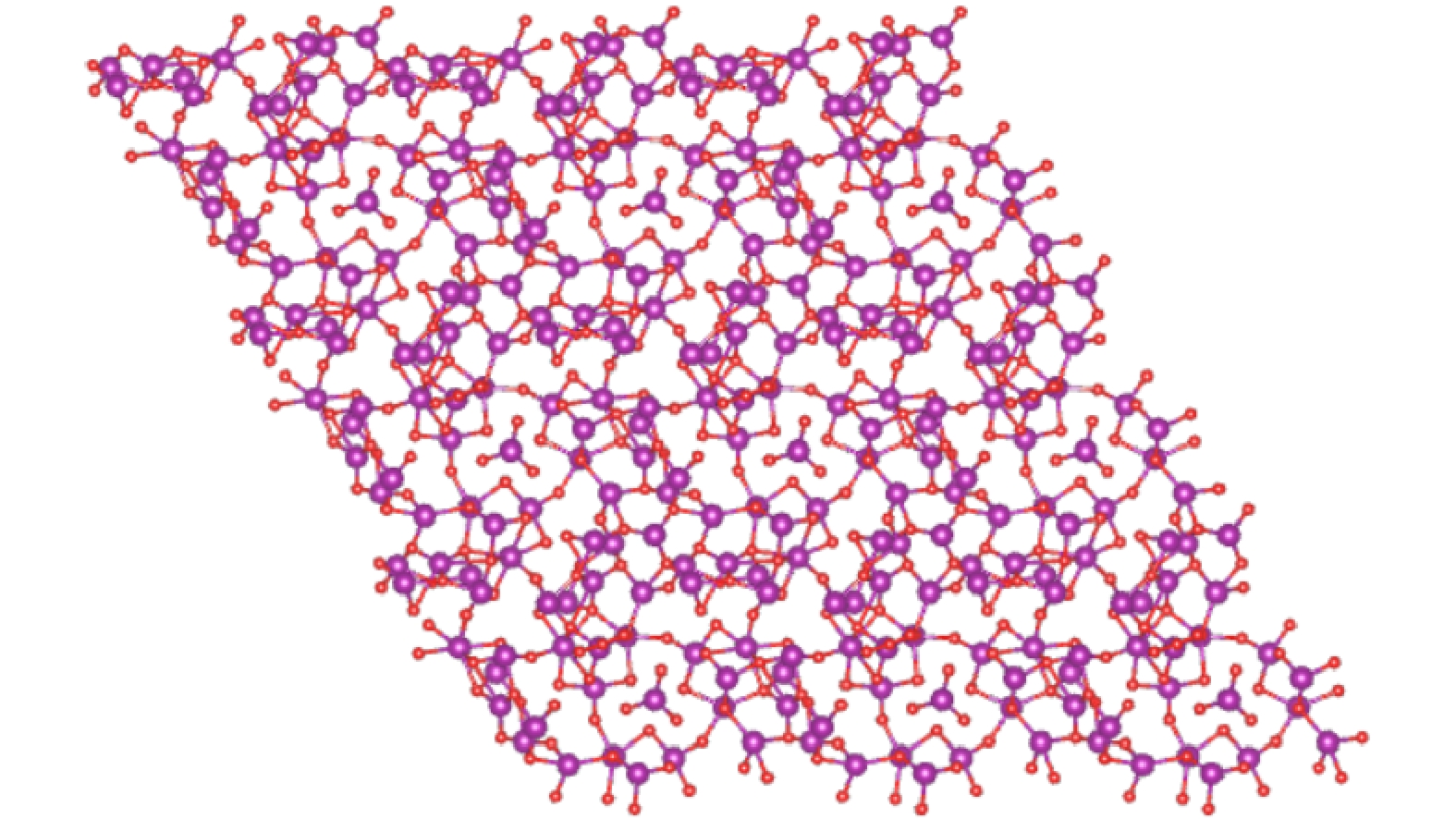
为分析催化剂的催化机理,使用密度泛函理论(density functional theory, DFT)模型计算了臭氧在Mn2O3晶体上的吸附过程。Mn2O3采用了最常见的(222)晶面,切面时,将其厚度设为1。单晶面包含43个单元,其中氧原子27个、锰原子16个。为避免表面间的原子相互作用,添加了2.4 nm的真空层。最终产生Mn2O3(222)晶面的模型,其晶格三维长度分别为a=1.330 77 nm, b=1.330 77 nm, c=2.50 nm,其3×3×1的超晶胞如图3所示。
在DFT计算过程中,采用原子PAW_PBE泛函,布里渊区k值设定为k=2×2×1。每一步运算都通过VASP 5.4.1 for Linux软件进行结构优化计算。运算采用的超算服务器,CPU为Intel Xeon Platinum单节点96核。
-
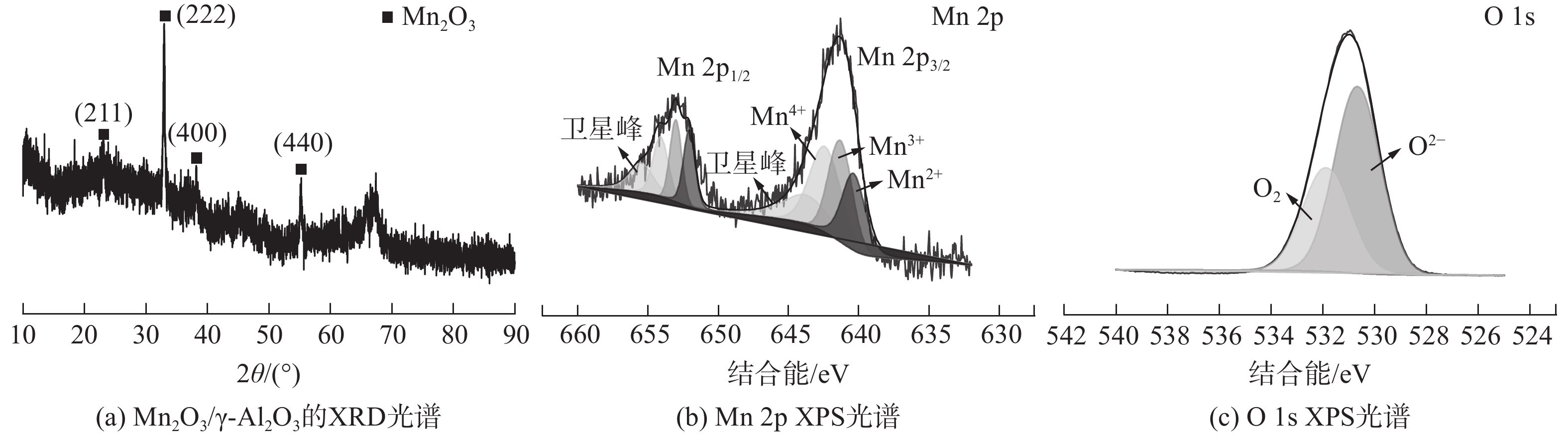
实验制备MnOx/γ-Al2O3的XRD如图4(a)所示。在2θ为23°、33°、38°和55°处出现了较强的衍射峰,这4个衍射峰可较好地对应Mn2O3晶体立方结构(PDF 002-0896)的(211)、(222)、(400)和(440)晶面。其中,2θ为33°和55°是Mn2O3的主峰。这表明Mn2O3在γ-Al2O3上具有良好的分散性[25]。
图4(b)为Mn2p的XPS图谱,其中2个分别位于641.7 eV和653.4 eV的主峰与文献中的Mn2O3所对应的峰值相匹配。对XPS图谱进行高斯拟合后,位于642.5 eV、641.5 eV和640.4 eV处的3个峰值分别对应于Mn4+、Mn3+和Mn2+。在643.8 eV处的最低峰值是卫星峰值,这是由于电荷从外层电子壳层转移到能量较高的空轨道所致。O1s的XPS图谱如图4(c)所示。位于530.7 eV处的峰可归因于晶格氧(O2-)与Mn的结合,而位于531.9 eV处的峰可归因于表面吸附氧(O2)。
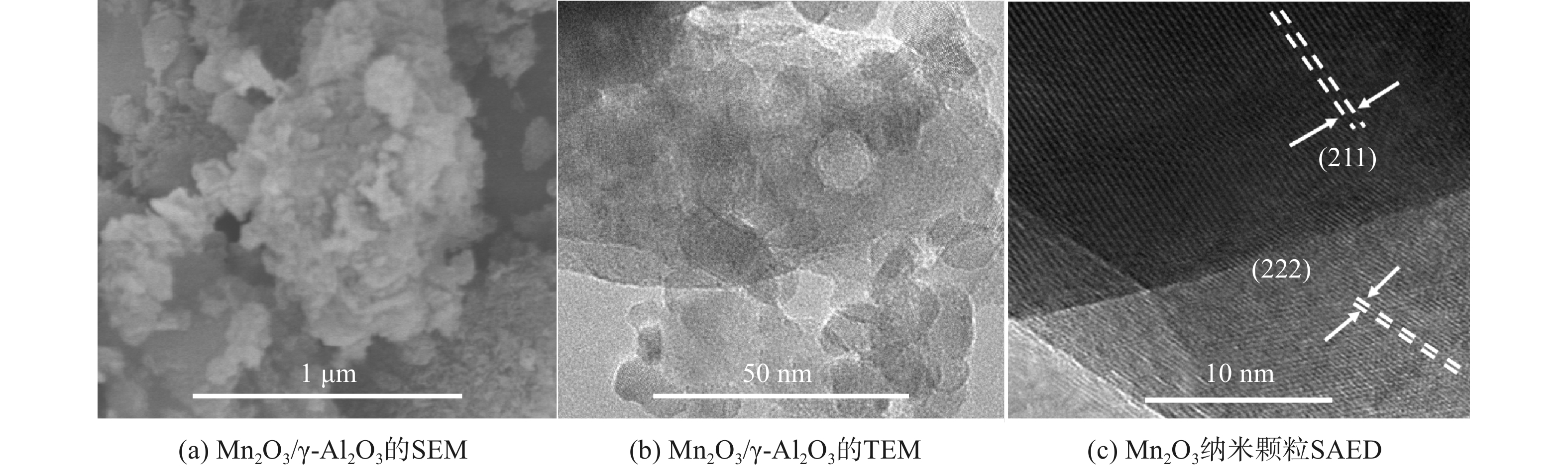
图5为Mn2O3/γ-Al2O3的SEM图像、TEM图像及选区电子衍射(selected area electron diffraction, SAED)图像。Mn2O3主要在γ-Al2O3表面以球形颗粒形式存在,且均匀分散在γ- Al2O3表面。Mn2O3的粒径约为10~100 nm。表面高度分散的Mn2O3晶体可促进催化过程中VOCs分子与催化剂间的接触。这可能会促进催化反应,最终促进VOCs的降解[26]。Mn2O3晶体呈立方结构与XRD结果一致。通过选区电子衍射分析获得Mn2O3的米勒指数为(211)、(222)、(400)和(440),与XRD分析中提到的一致。在图5(c)中截取的区域可观察到图3(b)中Mn2p的XPS光谱中2个主峰对应的2个晶面:(211)和(222)晶面,其晶面间距分别为0.386 nm和0.272 nm。
-
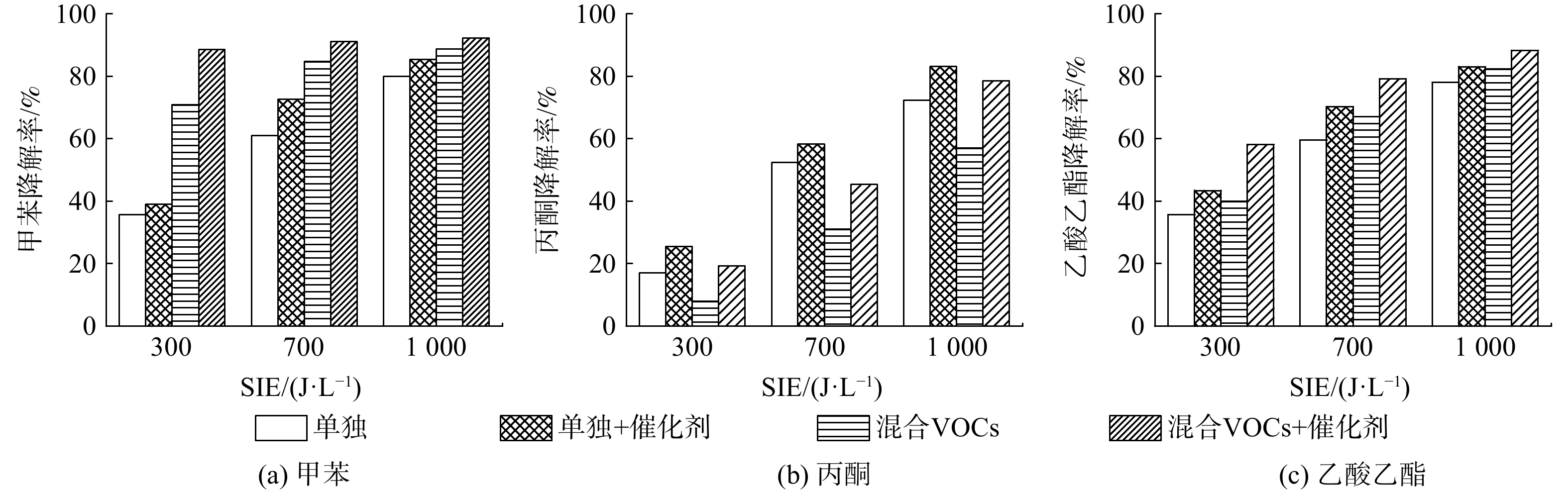
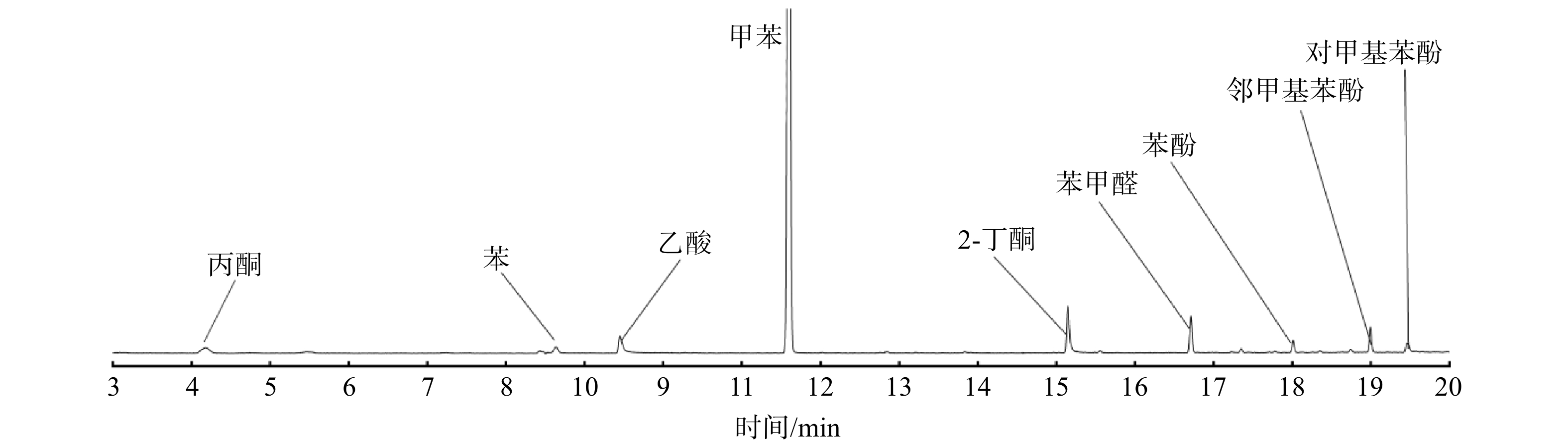
各降解条件下VOCs降解率如图6所示。甲苯、丙酮和乙酸乙酯的降解率均随SIE上升而上升,这与已有研究的结果一致。这是由于3种VOCs的分子电离能和分子结构不同所决定的[13]。对比有无催化剂条件下VOC单独降解与混合气中VOCs降解的降解率(具体数值见表1),可发现混合气中甲苯的降解率相较甲苯单独降解时的降解率有明显提升。当SIE为700 J ∙ L−1时,甲苯单独降解时降解率为61%,而混合气中的甲苯降解率为84.7%,提升率为38.9%;而混合气中乙酸乙酯的降解率相较乙酸乙酯单独降解时的降解率也有所提升,同等SIE下提升率约为12.6%。不同的是,混合气中丙酮的降解率相较丙酮单独降解时的降解率发生了明显下降。在SIE为700 J ∙ L−1条件下,丙酮单独降解时降解率为50.1%,而混合气中丙酮降解率为31.1%,降低了37.9%。其原因可能是3种VOCs的分解产物之间存在协同效应。当等离子体中存在多种VOCs时,会比单种VOCs产生更多活性物种,如自由基等。这可能会更有效地促进VOCs分解,从而导致相对容易降解的甲苯和乙酸乙酯的降解率得到提升[27]。然而,如图7所示,除了丙酮本身较难降解外,其还是甲苯降解的有机副产物之一[28]。在混合气中,甲苯的降解率相比甲苯单独降解有了极大提升的同时,也导致其有机副产物中丙酮体积分数上升,最终导致混合气中丙酮降解率出现下降。
随着Mn2O3/γ-Al2O3催化剂的引入,无论是单独或是混合状态,各VOCs的降解率均得以显著提升。当SIE为700 J ∙ L−1时,甲苯、乙酸乙酯及丙酮单独降解的降解率分别为61%、59.6%及50.1%;而在催化剂作用下,甲苯、乙酸乙酯以及丙酮单独降解的降解率分别提升至72.6%、70.2%及58.4%,此时催化剂对其降解率的提升量分别为19%、17.9%及16.7%。而在混合气中,同等SIE下甲苯、乙酸乙酯及丙酮的降解率分别为84.7%、67.1%及31.1%;在催化剂作用下,混合气中甲苯降解率被提升至91.1%,提升率约为7.5%;乙酸乙酯降解率被提升至79.2%,提升率约为18%;而丙酮的降解率被提升至45.3%,提升率为45.8%。此外,根据同等SIE下混合气中各VOCs的降解率可发现3种VOCs在混合气中的降解难度存在较大差距,甲苯、乙酸乙酯及丙酮的降解难度呈降序排列。这与前面单独降解的情况一致,表明混合和催化剂均不会改变VOCs的降解难易程度,从而说明电离能和分子结构是影响降解效率的重要因素。而Mn2O3/γ-Al2O3催化剂对混合气中甲苯、乙酸乙酯及丙酮降解率的提升效果随VOCs降解难度的上升而更加显著。
-
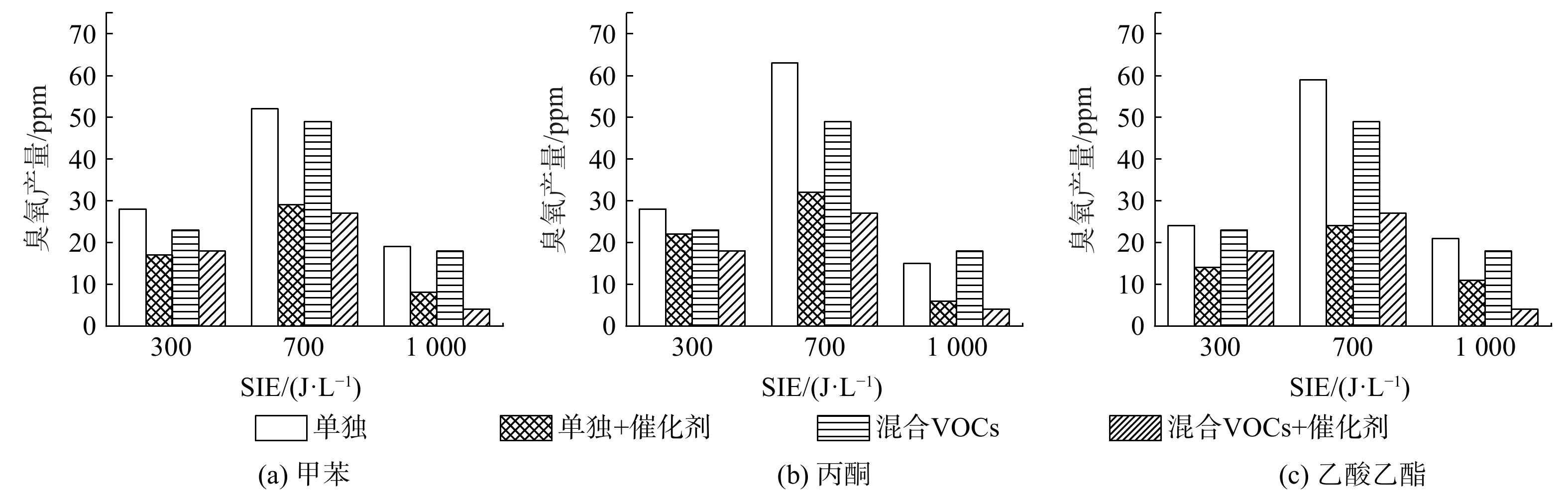
甲苯、丙酮、乙酸乙酯单独降解,以及混合VOCs降解过程中的臭氧产量如图8所示。在各种条件下,VOCs降解过程中的臭氧产量均随SIE上升呈先升后降趋势。如式(5)~(6)所示,臭氧的形成可分为2部分:高能电子与氧分子发生非弹性碰撞,形成氧原子;氧原子和氧分子在第三体的参与下生成臭氧[29]。
随着SIE上升,等离子体的电子密度和电子能量都随之增加,氧气分子与高能电子发生碰撞的几率随之上升,从而导致更多氧原子的产生,进而导致臭氧产量的上升。
然而,随着SIE的进一步上升,反应器腔体的温度也随之升高。STANISLAV等[30]发现反应器腔体温度的上升会导致臭氧产量的降低。随着Mn2O3/γ-Al2O3催化剂的引入,各条件下臭氧产量均出现明显降低。MnOx催化剂对臭氧生成有明显抑制作用[31],在混合VOCs中,这一抑制作用同样表现出色,并未因待降解气体成分的改变而表现异常。同时,混合VOCs中的臭氧产量相较3种VOCs单独降解时均有微弱下降。混合VOCs中VOCs总浓度的上升,将使更多氧原子参与VOCs及其中间产物的降解,从而使参加与O2发应生成臭氧的氧原子减少,即臭氧浓度比单种VOC降解时更少[4]。另外,VOCs体积分数的上升也会导致降解中间产物的增多,部分臭氧在深度氧化这些中间产物的过程中被消耗。
-
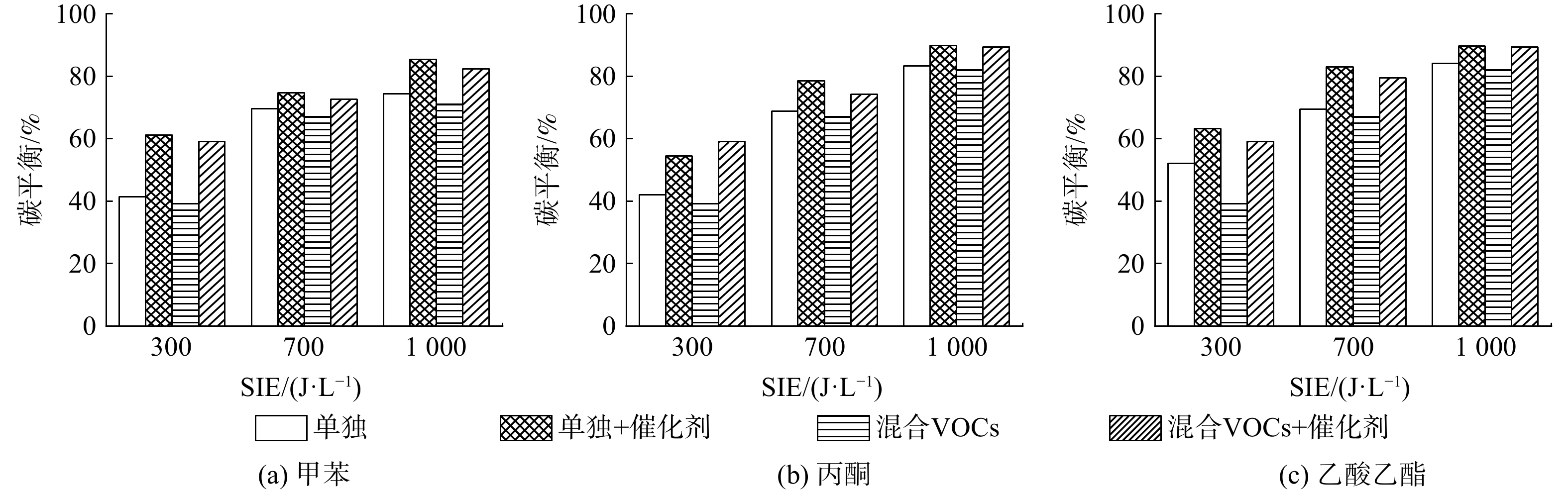
甲苯、丙酮、乙酸乙酯单独降解及混合VOCs降解的碳平衡情况如图9所示。随着SIE的上升,各条件下VOCs降解的碳平衡均呈上升趋势。此时,电场强度被增强,电子能量和密度也随之增强,进而提升了其与VOCs分子及VOCs分子降解中间产物碰撞的几率,从而导致碳平衡上升。此外,混合VOCs的碳平衡相较VOCs单独降解时的碳平衡均有一定程度下降。如,在SIE为700 J ∙ L−1时,甲苯单独降解的碳平衡为69.6%,丙酮单独降解的碳平衡为68.8%,乙酸乙酯单独降解的碳平衡为69.5%。而混合VOCs降解的碳平衡为67.1%,略有下降。相比VOCs单独降解,在混合VOCs中,由于VOCs体积分数的上升,部分等离子体放电产生的高能电子被用于甲苯、丙酮、乙酸乙酯分子的降解,被用于深度降解中间产物的高能电子数量则相应减少。最终导致混合VOCs降解的碳平衡较VOCs单独降解时有所降低。
随着Mn2O3/γ-Al2O3催化剂的引入,各条件下VOCs降解的碳平衡均得以提升。臭氧在催化剂表面可分解为氧分子和具有强氧化性的氧原子。氧原子除了可降解等离子体阶段中未降解的一部分VOCs外,还可将等离子体降解VOCs的中间产物深度氧化为COx和H2O。最终导致催化剂引入后的碳平衡得以提升。
-
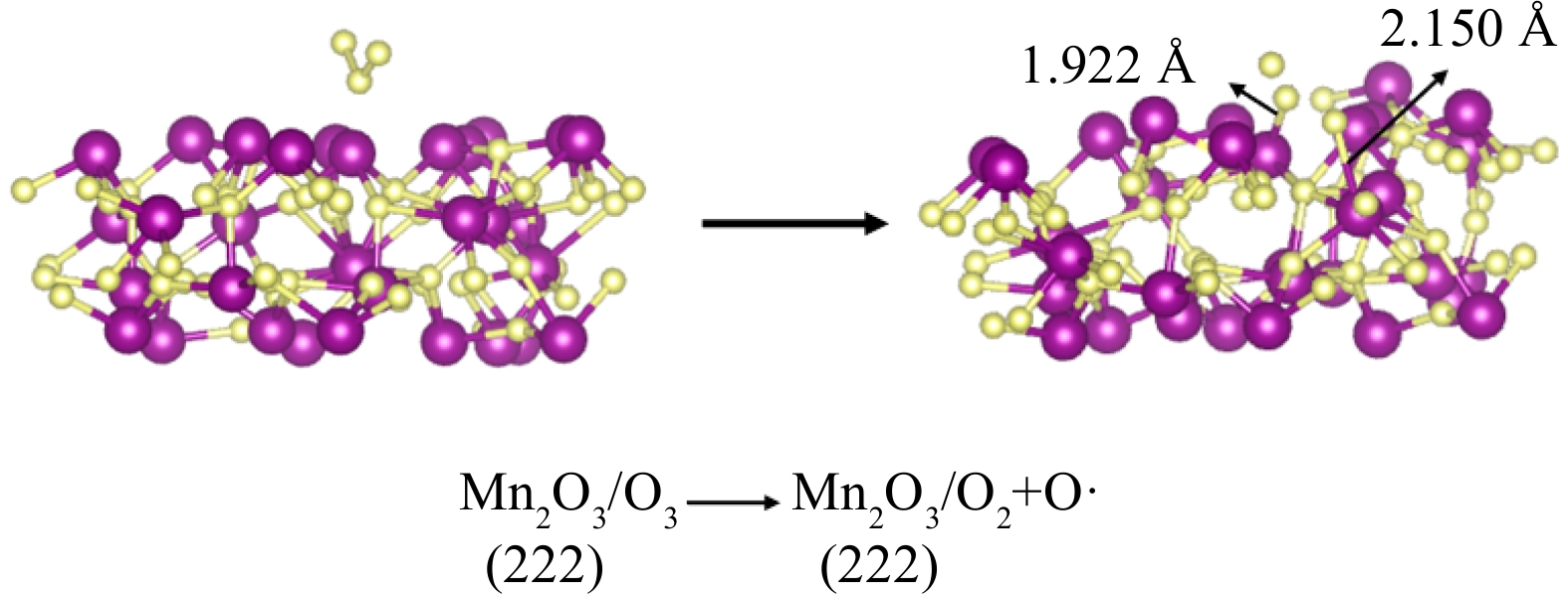
计算结果表明,O3中的2个O—O键长分别为0.128 9 nm、0.128 8 nm,键角为:118.1°。与实验结果得到的0.127 8 nm、0.127 8 nm、116.8°相比,误差分别为0.84%、0.80%、1.13%。误差极小表明选取的计算参数进行结构优化后,得到的参数处于可接受范围。对Mn2O3催化剂吸附O3的情形进行DFT计算,从而对O—Mn原子连接的方式进行研究。首先将优化后的O3分子模型和Mn2O3(222)晶面模型进行合并,并将O3分子置于晶面中一个Mn原子的正上方(图10)。
通过计算得到其吸附能为−16.64 eV。O3中的2个O原子分别吸附在了2个Mn原子上,其中O—Mn键的长度为0.192 2、0.215 0 nm。同时,3个O原子间的距离增加,分别为0.201 0 nm、0.194 4 nm。另一个O原子形成孤立离子形态,使其氧化性大大增强。在吸附的2个Mn原子附近Mn—O键分别从(0.185 9 nm、0.186 9 nm、0.187 0 nm)、(0.186 6 nm、0.193 0 nm、0.189 2 nm、0.211 0 nm)变为了(0.187 1 nm、0.196 9 nm、0.204 8 nm)、(0.194 5 nm、0.222 7 nm、0.202 8 nm、0.258 6 nm),其中1个O原子的距离为0.258 6 nm,可视为强氧化性的孤立氧原子。这也表明O3的吸附对Mn2O3晶面的表面结构产生了影响,吸附属于化学吸附。
另外,本研究还计算了O3分子的马利肯电荷(Maliken charge),其吸附前后的电荷如表2所示。在O3吸附于Mn2O3晶面的过程中,3个原子分别获得1.03 e、1.14 e、1.09 e的电子。因此,为使电负性平衡,O原子将被作为电子供体,其氧化性大大增强。O3吸附于Mn2O3催化剂表面,对催化剂活性有很大影响。在反应过程中,O3吸附于Mn2O3的222切面中,通常是O原子与Mn原子进行相互连接,吸附于Mn原子表面,从而改变了原切面的结构,从而增强其催化作用。
-
1) 对比单种VOCs与混合VOCs降解的降解率,可发现混合VOCs中甲苯的降解率相较单纯甲苯降解时的降解率有明显提升。乙酸乙酯的降解率相较单纯乙酸乙酯降解时的降解率略有提升。而丙酮的降解率相较单纯丙酮降解时的降解率却发生了明显下降。
2) VOCs降解过程中的臭氧产量均随SIE上升呈先升后降的趋势。同时,混合VOCs中的臭氧产量相较3种VOCs单独降解时均有微弱下降。Mn2O3/γ-Al2O3催化剂对于臭氧的生成有明显抑制作用。
3) 混合VOCs降解相较单种VOCs降解时的碳平衡均有一定程度下降。Mn2O3/γ-Al2O3催化剂的引入使得各条件下VOCs降解的碳平衡均得以提升。
4) Mn2O3/γ-Al2O3催化剂在协同低温等离子体降解多组分VOCs气体过程中,对混合VOCs中甲苯、乙酸乙酯及丙酮降解率的提升效果随VOCs种类降解难度的上升而更显著。
5) 通过DFT计算了O3在Mn2O3催化剂表面的吸附。O3吸附于Mn2O3的222切面中,通常是O原子与Mn原子进行相互连接,吸附于Mn原子表面,从而改变了原切面结构,增强了其催化作用。
催化剂协同介质阻挡放电等离子体对不同VOCs的催化选择性
Catalytic selectivity of catalyst in the degradation of mixed VOCs by dielectric barrier discharge plasma
-
摘要: 为考察混合气体中各组分对VOCs降解的影响,以及催化剂在协同低温等离子体降解多组分VOCs气体中的表现,选取甲苯、丙酮及乙酸乙酯组成混合VOCs进行低温等离子体降解,进而研究混合降解方式对混合VOCs气体各组分降解效果的影响。先制备了Mn2O3/γ-Al2O3催化剂,采用催化剂后置方式研究催化剂在协同低温等离子体降解多组分混合VOCs气体过程中的表现。结果表明:多组分混合VOCs降解时,甲苯和乙酸乙酯的降解率相较单独降解时都有所提升,当特定输入能量(SIE)为700 J∙L−1时,提升率分别为69.1%和12.64%,而丙酮的降解率相较单独降解时却发生了明显下降,下降了40.74%;多组分混合VOCs降解时的臭氧产量相较3种VOCs单独降解时均有微弱下降;多组分混合VOCs相较单种VOCs降解时的碳平衡均略有下降;在协同低温等离子体降解多组分VOCs气体过程中,Mn2O3/γ-Al2O3催化剂对混合VOCs中甲苯、乙酸乙酯及丙酮降解率有明显提升,且随VOCs降解难度的上升而更加明显,并使得各条件下VOCs降解的碳平衡均得到了提升。本研究结果可为低温等离子体降解VOCs的实际应用提供参考。Abstract: At present, there are few studies on the catalytic degradation of multi-component VOCs. The effect of gas component mixing on the degradation of each component is still unclear. The performance of the catalyst in the synergistic degradation of multi-component VOCs by non-thermal plasma also needs to be further studied. In this paper, the mixed VOCs composed of toluene, acetone and ethyl acetate were degraded by non-thermal plasma to investigate the degradation of each component in multi-component VOCs. Mn2O3/γ-Al2O3 catalyst was prepared, and the post catalyst method was applied to study the performance of the catalyst in the process of synergistic non-thermal plasma degradation of multi-component VOCs. The results showed that: 1) compared with single VOCs, the degradation rates of toluene and ethyl acetate in the degradation of mixed VOCs were higher than those in the degradation of pure toluene or ethyl acetate. When SIE was 700 J∙L−1, the enhancement rates were 69.1% and 12.64% respectively, while the degradation rate of acetone decreased significantly by 40.74%; 2) Compared with the degradation of three VOCs alone, the ozone production of mixed VOCs’ degradation decreased slightly; 3) Compared with single VOCs, the carbon balance of mixed VOCs’ degradation decreased slightly as well; 4) In the process of synergistic non-thermal plasma degradation of multi-component VOCs by Mn2O3/γ-Al2O3 catalyst, the improvement rate of degradation rate of toluene, ethyl acetate and acetone by catalyst in mixed VOCs increased with the increase of degradation difficulty of VOCs. The carbon balance of VOCs degradation under various conditions was improved by catalyst.
-
可挥发性有机化合物(volatile organic compounds, VOCs)是大气污染物中一大类[1-3]。浓度较高的VOCs气体会刺激人的眼睛、鼻子或咽喉等,导致干咳头晕、恶心疲劳等症状。长期生活在受VOCs污染的环境中,人体的神经系统会被损害,并诱发癌症,故VOCs的治理刻不容缓[4-6]。
传统VOCs处理技术主要有燃烧法、催化氧化法、吸收吸附法等。其中,燃烧法的操作较为简单,但因危险性较高,故对安全防护的要求较高;催化氧化法不需要额外试剂,且产生污染物较少,但同时存在催化剂稳定性和寿命等限制[7-8];吸收法可将VOCs回收再利用,但需根据待处理VOCs种类使用特定吸收剂,普适性较差;吸附法常用活性炭作为吸附剂,净化率高,但活性炭使用寿命很短,需频繁更换。
低温等离子体(non-thermal plasma, NTP)技术是一种新型VOCs处理技术,相较于传统VOCs处理技术,具有适用性广、响应快速等特点,因而受到广泛关注[9-11]。在众多产生NTP的放电形式中,介质阻挡放电(dielectric barrier discharge, DBD)因其结构简单、可通过改变放电参数调控等离子体能量密度,且能处理较大流量气体等优势而被广泛研究。王保伟等[12]通过研究放电间距对单介质阻挡放电(single dielectric barrier discharge, SDBD) 等离子体降解甲苯的影响,发现随放电间距的增大,甲苯转化率和CO2选择性呈先增后降趋势。ZHAO等[13]使用双介质阻挡放电(double dielectric barrier discharge, DDBD)等离子体降解多种芳烃、烷烃、酮和酯类VOCs,发现电离能是影响所有VOCs降解效率的重要参数,电离能越大,降解效率越低。相较于SDBD放电腔,DDBD放电腔可很好地保护放电电极不受工作气体污染。
为进一步优化NTP技术,提升VOCs转化率,并降低NTP降解VOCs过程中产生的臭氧与有机副产物产量,催化剂协同技术被越来越多应用于NTP降解VOCs的体系中[10-11, 14-17]。在众多研究中,对催化剂性能的表征大多使用单种VOCs进行。然而,实际情况下待处理的VOCs组分复杂,催化剂在多组分VOCs的处理中的表现还鲜有报道。
本研究拟使用双介质阻挡放电(DDBD)反应器产生低温等离子体,以甲苯、丙酮及乙酸乙酯的混合气体作为待降解模拟VOCs混合废气[18-19],并制备常用于协同NTP降解VOCs的Mn2O3/γ-Al2O3催化剂,以研究NTP降解复杂成分VOCs的特性,以及催化剂对NTP降解混合VOCs的影响,以期为NTP降解VOCs的实际应用提供参考。
1. 实验部分
1.1 实验装置
实验装置及流程图如图1所示。模拟混合VOCs废气由高浓度丙酮、甲苯及乙酸乙酯标气经稀释得到。稀释标气所用气体为经过纯净空气发生器干燥后的压缩空气。使用4个质量流量控制器(mass flow controller, MFC)分别控制丙酮、甲苯、乙酸乙酯及压缩空气的流量,以得到实验所需的各VOCs组分的初始浓度。模拟混合VOCs经缓冲瓶混合后通入DDBD反应器降解,模拟VOCs的流速固定在1 L ∙ min−1。在VOCs单独降解实验中,甲苯、丙酮及乙酸乙酯的初始体积分数均为(33±2)×10−6。在混合VOCs降解实验中,甲苯、丙酮及乙酸乙酯的初始体积分数也均为(33±2)×10−6。
DDBD反应器的2层介质分别为1根外径为20 mm,内径为17 mm的石英管(外管),以及1根外径为8 mm、内径为6 mm的石英管(内管)。内管中放置1根直径6 mm的铜棒作为高压电极,外管缠绕宽度为10 cm的铝箔作为接地电极。模拟混合VOCs经DDBD反应器进气口进入反应器内进行低温等离子体降解。经初步降解后的废气由反应器出气口进入催化剂反应管进行进一步反应。该催化剂反应管为内径5 mm、长度30 cm的石英管。
降解前后的VOCs、CO和CO2体积分数均使用气相色谱仪(GC2060ⅢA,上海锐敏仪器有限公司)在线测定。其中,VOCs的体积分数使用配置有HT-5型毛细管柱(柱长30 m,内径0.32 mm)的火焰离子化检测器(flame ionization detector, FID)检测;CO和CO2的体积分数使用配有甲烷化转化炉的FID检测器检测。气相色谱仪的检测条件设定为:炉温60 ℃,检测器温度140 ℃,进样器温度120 ℃,甲烷转换炉320 ℃。反应过程中生成的臭氧体积分数使用臭氧检测仪(GT-2000-k3, Korno)测定。
1.2 催化剂的制备
将一定量的Mn(NO3)2(AR,国药集团化学试剂有限公司)与2 g γ-Al2O3(球形,国药集团化学试剂有限公司)分散在含有分散剂聚乙烯吡咯烷酮(质量分数2%)(AR,国药集团化学试剂有限公司)、乙醇(质量分数12%)(AR,国药集团化学试剂有限公司)和去离子水的混合溶液中。其中,Mn(NO3)2的量取决于Mn元素与γ-Al2O3的质量比。将混合物超声分散1 h后转移进容积为100 mL的聚四氟乙烯瓶中,在140 ℃条件下放置6 h[20-21]。混合物冷却至室温后,用去离子水洗涤3次,并在60 ℃下干燥,最后在马弗炉中以500 ℃煅烧产物6 h以获得催化剂。
1.3 数据的统计分析
VOCs废气的降解效果通常使用降解率与碳平衡进行表征。其中,VOCs的降解率(degradation rate, DR)由式(1)计算得到[22]。
DR=cin−coutcin×100% (1) 式中:
cin cout VOCs降解后的碳平衡(carbon balance, CB)可通过式(2)计算得到。
CB=nCO+nCO27×nT+4×nE+3×nA (2) 式中:
nCO nCO2 nT nE nA DDBD放电腔通过高压电源(CTP-2000K,南京苏曼电子有限公司)驱动放电,电源频率为10 kHz;放电腔两端的电压和电流分别通过高压探头( P6015A, Tektronix)及电流探头(5315, ETA)检测,并使用数字示波器记录(MDO3032, Tektronix)记录其放电波形。本课题组前期研究发现,调制脉冲电源可改善DDBD等离子体降解VOCs的能量效率。因此,在本实验中,电源通过一个矩形脉冲来调制一个中心频率为10 kHz 的正弦波形,并将高压电源的占空比和调制频率固定为20%与150 Hz。调制后的DDBD放电典型电流电压波形如图2所示。
反应器放电功率P可通过式(3)计算得到[23]。
P=f∫T0U(t)I(t)dt (3) 式中:T为脉冲电源的脉冲宽度,s;f为调制脉冲频率,Hz; U(t)为高压探头测得的放电电压,V;I(t)为电流探针测得的放电电流,A。
进而可通过式(4)计算得到低温等离子体降解VOCs过程中的特定输入能量(specific input energy,SIE)。SIE是低温等离子体降解VOCs效果评价的重要参数之一[24]。
SIE=PQ×60 (4) 式中:Q为模拟VOCs废气的流速,L ∙ min−1。
1.4 催化剂表征
催化剂的元素含量通过Prodigy ICP装置(利曼,美国)上的电感耦合等离子体(inductively coupled plasma, ICP)光电发射光谱进行测量。氮气吸附-脱附等温线在ASAP-2460分析仪上获得的。使用传统brunauer-emmett-teller(BET)和barrett-joyner-halenda(BJH)方程中的吸附数据确定催化剂的比表面积、孔径分布和孔体积;使用DD Max-2550PC型18 kW转靶X射线衍射仪(里加库,日本)记录催化剂粉末X射线衍射(X-ray diffraction, XRD)图;催化剂的X射线光电子能谱(X-ray photoelectron spectroscopy, XPS)由Thermo Escalab 250Xi型X射线光电子能谱仪(ThermoFisher,美国)在Al-K(1486.6 eV,150 W)辐射下获得;通过扫描电镜(scanning electron microscope, SEM)(JEOL 7800 F,日本)研究催化剂的形态。使用高分辨率透射电镜(high resolution transmission electron microscope, HR-TEM)(FEI Tecnai G2F30,美国)测定了催化剂的结构和元素图。
1.5 DFT计算方法
为分析催化剂的催化机理,使用密度泛函理论(density functional theory, DFT)模型计算了臭氧在Mn2O3晶体上的吸附过程。Mn2O3采用了最常见的(222)晶面,切面时,将其厚度设为1。单晶面包含43个单元,其中氧原子27个、锰原子16个。为避免表面间的原子相互作用,添加了2.4 nm的真空层。最终产生Mn2O3(222)晶面的模型,其晶格三维长度分别为a=1.330 77 nm, b=1.330 77 nm, c=2.50 nm,其3×3×1的超晶胞如图3所示。
在DFT计算过程中,采用原子PAW_PBE泛函,布里渊区k值设定为k=2×2×1。每一步运算都通过VASP 5.4.1 for Linux软件进行结构优化计算。运算采用的超算服务器,CPU为Intel Xeon Platinum单节点96核。
2. 结果与讨论
2.1 催化剂表征
实验制备MnOx/γ-Al2O3的XRD如图4(a)所示。在2θ为23°、33°、38°和55°处出现了较强的衍射峰,这4个衍射峰可较好地对应Mn2O3晶体立方结构(PDF 002-0896)的(211)、(222)、(400)和(440)晶面。其中,2θ为33°和55°是Mn2O3的主峰。这表明Mn2O3在γ-Al2O3上具有良好的分散性[25]。
图4(b)为Mn2p的XPS图谱,其中2个分别位于641.7 eV和653.4 eV的主峰与文献中的Mn2O3所对应的峰值相匹配。对XPS图谱进行高斯拟合后,位于642.5 eV、641.5 eV和640.4 eV处的3个峰值分别对应于Mn4+、Mn3+和Mn2+。在643.8 eV处的最低峰值是卫星峰值,这是由于电荷从外层电子壳层转移到能量较高的空轨道所致。O1s的XPS图谱如图4(c)所示。位于530.7 eV处的峰可归因于晶格氧(O2-)与Mn的结合,而位于531.9 eV处的峰可归因于表面吸附氧(O2)。
图5为Mn2O3/γ-Al2O3的SEM图像、TEM图像及选区电子衍射(selected area electron diffraction, SAED)图像。Mn2O3主要在γ-Al2O3表面以球形颗粒形式存在,且均匀分散在γ- Al2O3表面。Mn2O3的粒径约为10~100 nm。表面高度分散的Mn2O3晶体可促进催化过程中VOCs分子与催化剂间的接触。这可能会促进催化反应,最终促进VOCs的降解[26]。Mn2O3晶体呈立方结构与XRD结果一致。通过选区电子衍射分析获得Mn2O3的米勒指数为(211)、(222)、(400)和(440),与XRD分析中提到的一致。在图5(c)中截取的区域可观察到图3(b)中Mn2p的XPS光谱中2个主峰对应的2个晶面:(211)和(222)晶面,其晶面间距分别为0.386 nm和0.272 nm。
2.2 VOCs降解实验结果
2.2.1 VOCs单独降解与混合VOCs降解的降解率对比
各降解条件下VOCs降解率如图6所示。甲苯、丙酮和乙酸乙酯的降解率均随SIE上升而上升,这与已有研究的结果一致。这是由于3种VOCs的分子电离能和分子结构不同所决定的[13]。对比有无催化剂条件下VOC单独降解与混合气中VOCs降解的降解率(具体数值见表1),可发现混合气中甲苯的降解率相较甲苯单独降解时的降解率有明显提升。当SIE为700 J ∙ L−1时,甲苯单独降解时降解率为61%,而混合气中的甲苯降解率为84.7%,提升率为38.9%;而混合气中乙酸乙酯的降解率相较乙酸乙酯单独降解时的降解率也有所提升,同等SIE下提升率约为12.6%。不同的是,混合气中丙酮的降解率相较丙酮单独降解时的降解率发生了明显下降。在SIE为700 J ∙ L−1条件下,丙酮单独降解时降解率为50.1%,而混合气中丙酮降解率为31.1%,降低了37.9%。其原因可能是3种VOCs的分解产物之间存在协同效应。当等离子体中存在多种VOCs时,会比单种VOCs产生更多活性物种,如自由基等。这可能会更有效地促进VOCs分解,从而导致相对容易降解的甲苯和乙酸乙酯的降解率得到提升[27]。然而,如图7所示,除了丙酮本身较难降解外,其还是甲苯降解的有机副产物之一[28]。在混合气中,甲苯的降解率相比甲苯单独降解有了极大提升的同时,也导致其有机副产物中丙酮体积分数上升,最终导致混合气中丙酮降解率出现下降。
表 1 在SIE为700 J ∙ L−1时,各VOCs的降解率及其提升率Table 1. Degradation rate and improvement rate of VOCs at SIE of 700 J ∙ L−1指标 甲苯 乙酸乙酯 丙酮 单独 混合 单独 混合 单独 混合 无催化时的降解率 61% 84.7% 59.6% 67.1% 50.1% 31.1% 有催化时的降解率 72.6% 91.1% 70.2% 79.1% 58.4% 45.3% 提升率 19% 7.5% 17.9% 18% 16.7% 45.8% 随着Mn2O3/γ-Al2O3催化剂的引入,无论是单独或是混合状态,各VOCs的降解率均得以显著提升。当SIE为700 J ∙ L−1时,甲苯、乙酸乙酯及丙酮单独降解的降解率分别为61%、59.6%及50.1%;而在催化剂作用下,甲苯、乙酸乙酯以及丙酮单独降解的降解率分别提升至72.6%、70.2%及58.4%,此时催化剂对其降解率的提升量分别为19%、17.9%及16.7%。而在混合气中,同等SIE下甲苯、乙酸乙酯及丙酮的降解率分别为84.7%、67.1%及31.1%;在催化剂作用下,混合气中甲苯降解率被提升至91.1%,提升率约为7.5%;乙酸乙酯降解率被提升至79.2%,提升率约为18%;而丙酮的降解率被提升至45.3%,提升率为45.8%。此外,根据同等SIE下混合气中各VOCs的降解率可发现3种VOCs在混合气中的降解难度存在较大差距,甲苯、乙酸乙酯及丙酮的降解难度呈降序排列。这与前面单独降解的情况一致,表明混合和催化剂均不会改变VOCs的降解难易程度,从而说明电离能和分子结构是影响降解效率的重要因素。而Mn2O3/γ-Al2O3催化剂对混合气中甲苯、乙酸乙酯及丙酮降解率的提升效果随VOCs降解难度的上升而更加显著。
2.2.2 VOCs单独降解与混合VOCs降解的臭氧产量对比
甲苯、丙酮、乙酸乙酯单独降解,以及混合VOCs降解过程中的臭氧产量如图8所示。在各种条件下,VOCs降解过程中的臭氧产量均随SIE上升呈先升后降趋势。如式(5)~(6)所示,臭氧的形成可分为2部分:高能电子与氧分子发生非弹性碰撞,形成氧原子;氧原子和氧分子在第三体的参与下生成臭氧[29]。
e+O2→2O+e (5) O+O2+M→O3+M (6) 随着SIE上升,等离子体的电子密度和电子能量都随之增加,氧气分子与高能电子发生碰撞的几率随之上升,从而导致更多氧原子的产生,进而导致臭氧产量的上升。
然而,随着SIE的进一步上升,反应器腔体的温度也随之升高。STANISLAV等[30]发现反应器腔体温度的上升会导致臭氧产量的降低。随着Mn2O3/γ-Al2O3催化剂的引入,各条件下臭氧产量均出现明显降低。MnOx催化剂对臭氧生成有明显抑制作用[31],在混合VOCs中,这一抑制作用同样表现出色,并未因待降解气体成分的改变而表现异常。同时,混合VOCs中的臭氧产量相较3种VOCs单独降解时均有微弱下降。混合VOCs中VOCs总浓度的上升,将使更多氧原子参与VOCs及其中间产物的降解,从而使参加与O2发应生成臭氧的氧原子减少,即臭氧浓度比单种VOC降解时更少[4]。另外,VOCs体积分数的上升也会导致降解中间产物的增多,部分臭氧在深度氧化这些中间产物的过程中被消耗。
2.2.3 VOCs单独降解与混合VOCs降解的碳平衡对比
甲苯、丙酮、乙酸乙酯单独降解及混合VOCs降解的碳平衡情况如图9所示。随着SIE的上升,各条件下VOCs降解的碳平衡均呈上升趋势。此时,电场强度被增强,电子能量和密度也随之增强,进而提升了其与VOCs分子及VOCs分子降解中间产物碰撞的几率,从而导致碳平衡上升。此外,混合VOCs的碳平衡相较VOCs单独降解时的碳平衡均有一定程度下降。如,在SIE为700 J ∙ L−1时,甲苯单独降解的碳平衡为69.6%,丙酮单独降解的碳平衡为68.8%,乙酸乙酯单独降解的碳平衡为69.5%。而混合VOCs降解的碳平衡为67.1%,略有下降。相比VOCs单独降解,在混合VOCs中,由于VOCs体积分数的上升,部分等离子体放电产生的高能电子被用于甲苯、丙酮、乙酸乙酯分子的降解,被用于深度降解中间产物的高能电子数量则相应减少。最终导致混合VOCs降解的碳平衡较VOCs单独降解时有所降低。
随着Mn2O3/γ-Al2O3催化剂的引入,各条件下VOCs降解的碳平衡均得以提升。臭氧在催化剂表面可分解为氧分子和具有强氧化性的氧原子。氧原子除了可降解等离子体阶段中未降解的一部分VOCs外,还可将等离子体降解VOCs的中间产物深度氧化为COx和H2O。最终导致催化剂引入后的碳平衡得以提升。
2.2.4 臭氧在Mn2O3晶体上的吸附过程
计算结果表明,O3中的2个O—O键长分别为0.128 9 nm、0.128 8 nm,键角为:118.1°。与实验结果得到的0.127 8 nm、0.127 8 nm、116.8°相比,误差分别为0.84%、0.80%、1.13%。误差极小表明选取的计算参数进行结构优化后,得到的参数处于可接受范围。对Mn2O3催化剂吸附O3的情形进行DFT计算,从而对O—Mn原子连接的方式进行研究。首先将优化后的O3分子模型和Mn2O3(222)晶面模型进行合并,并将O3分子置于晶面中一个Mn原子的正上方(图10)。
通过计算得到其吸附能为−16.64 eV。O3中的2个O原子分别吸附在了2个Mn原子上,其中O—Mn键的长度为0.192 2、0.215 0 nm。同时,3个O原子间的距离增加,分别为0.201 0 nm、0.194 4 nm。另一个O原子形成孤立离子形态,使其氧化性大大增强。在吸附的2个Mn原子附近Mn—O键分别从(0.185 9 nm、0.186 9 nm、0.187 0 nm)、(0.186 6 nm、0.193 0 nm、0.189 2 nm、0.211 0 nm)变为了(0.187 1 nm、0.196 9 nm、0.204 8 nm)、(0.194 5 nm、0.222 7 nm、0.202 8 nm、0.258 6 nm),其中1个O原子的距离为0.258 6 nm,可视为强氧化性的孤立氧原子。这也表明O3的吸附对Mn2O3晶面的表面结构产生了影响,吸附属于化学吸附。
另外,本研究还计算了O3分子的马利肯电荷(Maliken charge),其吸附前后的电荷如表2所示。在O3吸附于Mn2O3晶面的过程中,3个原子分别获得1.03 e、1.14 e、1.09 e的电子。因此,为使电负性平衡,O原子将被作为电子供体,其氧化性大大增强。O3吸附于Mn2O3催化剂表面,对催化剂活性有很大影响。在反应过程中,O3吸附于Mn2O3的222切面中,通常是O原子与Mn原子进行相互连接,吸附于Mn原子表面,从而改变了原切面的结构,从而增强其催化作用。
表 2 O3分子的马利肯电荷Table 2. Maliken charge of O3 molecule原子种类 吸附前/e 吸附后/e O1 4.94 5.97 O2 4.94 6.09 O3 4.94 6.03 3. 结论
1) 对比单种VOCs与混合VOCs降解的降解率,可发现混合VOCs中甲苯的降解率相较单纯甲苯降解时的降解率有明显提升。乙酸乙酯的降解率相较单纯乙酸乙酯降解时的降解率略有提升。而丙酮的降解率相较单纯丙酮降解时的降解率却发生了明显下降。
2) VOCs降解过程中的臭氧产量均随SIE上升呈先升后降的趋势。同时,混合VOCs中的臭氧产量相较3种VOCs单独降解时均有微弱下降。Mn2O3/γ-Al2O3催化剂对于臭氧的生成有明显抑制作用。
3) 混合VOCs降解相较单种VOCs降解时的碳平衡均有一定程度下降。Mn2O3/γ-Al2O3催化剂的引入使得各条件下VOCs降解的碳平衡均得以提升。
4) Mn2O3/γ-Al2O3催化剂在协同低温等离子体降解多组分VOCs气体过程中,对混合VOCs中甲苯、乙酸乙酯及丙酮降解率的提升效果随VOCs种类降解难度的上升而更显著。
5) 通过DFT计算了O3在Mn2O3催化剂表面的吸附。O3吸附于Mn2O3的222切面中,通常是O原子与Mn原子进行相互连接,吸附于Mn原子表面,从而改变了原切面结构,增强了其催化作用。
-
表 1 在SIE为700 J ∙ L−1时,各VOCs的降解率及其提升率
Table 1. Degradation rate and improvement rate of VOCs at SIE of 700 J ∙ L−1
指标 甲苯 乙酸乙酯 丙酮 单独 混合 单独 混合 单独 混合 无催化时的降解率 61% 84.7% 59.6% 67.1% 50.1% 31.1% 有催化时的降解率 72.6% 91.1% 70.2% 79.1% 58.4% 45.3% 提升率 19% 7.5% 17.9% 18% 16.7% 45.8% 表 2 O3分子的马利肯电荷
Table 2. Maliken charge of O3 molecule
原子种类 吸附前/e 吸附后/e O1 4.94 5.97 O2 4.94 6.09 O3 4.94 6.03 -
[1] 赵琼. 低温等离子体降解VOCs的DBD反应器优化探索和产物分析[D]. 上海: 东华大学, 2017. [2] 赵琳, 张英锋, 李荣焕, 等. VOC的危害及回收与处理技术[J]. 化学教育, 2015, 36(16): 1-6. [3] 冯发达. 反电晕等离子体发生方法及协同催化处理挥发性有机物的研究[D]. 杭州: 浙江大学, 2014. [4] ZHANG H B, LI K, SHU C H, et al. Enhancement of styrene removal using a novel double-tube dielectric barrier discharge (DDBD) reactor[J]. Chemical Engineering Journal, 2014, 256: 107-118. doi: 10.1016/j.cej.2014.06.105 [5] NA C J, YOO M J, TSANG D C W, et al. High-performance materials for effective sorptive removal of formaldehyde in air[J]. Journal of Hazardous Materials, 2018, 366: 452-465. [6] CHUNG W C, MEI D H, TU X, et al. Removal of VOCs from gas streams via plasma and catalysis[J]. Catalysis Reviews-Science And Engineering, 2019, 61(2): 270-331. doi: 10.1080/01614940.2018.1541814 [7] LU J C, HAO H S, ZHANG L M, et al. The investigation of the role of basic lanthanum (La) species on the improvement of catalytic activity and stability of HZSM-5 material for eliminating methanethiol- (CH3SH)[J]. Applied Catalysis B Environmental, 2018, 237: 185-197. doi: 10.1016/j.apcatb.2018.05.063 [8] LU J C, LIU J P, ZHAO Y T, et al. The identification of active chromium species to enhance catalytic behaviors of alumina-based catalysts for sulfur-containing VOC abatement[J]. Journal of Hazardous Materials, 2020, 384: 121289. doi: 10.1016/j.jhazmat.2019.121289 [9] 吴萧, 刘盛余, 何廷宇, 康岷慧, 等. 介质阻挡放电低温等离子体技术处理3种代表性VOC[J]. 环境工程学报, 2017, 11(10): 5502-5508. doi: 10.12030/j.cjee.201612064 [10] 秦彩虹, 党小庆, 黄家玉, 等. 气体循环条件下等离子体催化氧化吸附态的苯和甲苯[J]. 环境工程学报, 2017, 11(3): 1691-1697. doi: 10.12030/j.cjee.201512014 [11] 陈睿, 王升高, 崔丽佳, 等. FeMnOx和等离子体对苯的协同降解[J]. 真空与低温, 2016, 22(3): 173-176. doi: 10.3969/j.issn.1006-7086.2016.03.012 [12] 王保伟, 姚淑美, 彭叶平, 等. 介质阻挡放电等离子体降解高浓度甲苯[J]. 环境工程学报, 2018, 12(7): 1977-1985. doi: 10.12030/j.cjee.201801156 [13] ZHAO X L, LIU X, J LIU, et al. The effect of ionization energy and hydrogen weight fraction on the non-thermal plasma VOCs removal efficiency[J]. Journal of Physics D:Applied Physics, 2019, 52: 145201. doi: 10.1088/1361-6463/aafe8b [14] 郭玉芳, 叶代启, 陈克复. 挥发性有机化合物(VOCs)的低温等离子体-催化协同净化[J]. 工业催化, 2005(11): 4-8. [15] WU Z L, ZHOU W L, ZHU Z B, et al. Enhanced oxidation of xylene using plasma activation of an Mn/Al2O3 catalyst[J]. IEEE Transactions on Plasma Science, 2020, 48(1): 163-172. doi: 10.1109/TPS.2019.2959698 [16] ZHU X B, TU X, MEI D H, et al. Investigation of hybrid plasma-catalytic removal of acetone over CuO/γ-Al2O3 catalysts using response surface method[J]. Chemosphere, 2016, 155: 9-17. doi: 10.1016/j.chemosphere.2016.03.114 [17] ZHU X B, ZHANG S, YANG Y, et al. Enhanced performance for plasma-catalytic oxidation of ethyl acetate over La1-xCexCoO3+δ catalysts[J]. Applied Catalysis B-Environmental, 2017, 213: 97-105. doi: 10.1016/j.apcatb.2017.04.066 [18] LU W J, ABBAS Y, MUSTAFA M F, et al. A review on application of dielectric barrier discharge plasma technology on the abatement of volatile organic compounds[J]. Frontiers of Environmental Science & Engineering, 2019, 13(2): 30. [19] JIANG N, GUO L J, QIU C, et al. Reactive species distribution characteristics and toluene destruction in the three-electrode DBD reactor energized by different pulsed modes[J]. Chemical Engineering Journal, 2018, 350: 12-19. doi: 10.1016/j.cej.2018.05.154 [20] ZHAO J H, NING L, YU R X, et al. Magnetic field enhanced denitrification in nitrate and ammonia contaminated water under 3D/2D Mn2O3/γ-C3N4 photocatalysis[J]. Chemical Engineering Journal, 2018, 349: 530-538. doi: 10.1016/j.cej.2018.05.124 [21] ZHANG X D, LV X T, BI F K, et al. Highly efficient Mn2O3 catalysts derived from Mn-MOFs for toluene oxidation: The influence of MOFs precursors[J]. Molecular Catalysis, 2019, 482: 110701. [22] KARUPPIAH J, REDDY E L, REDDY P M K, et al. Abatement of mixture of volatile organic compounds (VOCs) in a catalytic non-thermal plasma reactor[J]. Journal of Hazardous Materials, 2012, 237: 283-289. [23] MA T P, ZHAO Q, LIU J Q, et al. Study of humidity effect on benzene decomposition by the dielectric barrier discharge nonthermal plasma reactor[J]. Plasma Science & Technology, 2016, 18(6): 686-692. [24] VANDENBROUCKE A M, MORENT R, DE GEYTER N, et al. Non-thermal plasma for non-catalytic and catalytic VOC abatement[J]. Journal of Hazardous Materials, 2011, 195: 30-45. doi: 10.1016/j.jhazmat.2011.08.060 [25] XIE S H, DENG J G, LIU Y X, et al. Excellent catalytic performance, thermal stability, and water resistance of 3DOM Mn2O3-supported Au-Pd alloy nanoparticles for the complete oxidation of toluene[J]. Applied Catalysis, A. General, 2015, 507: 82-90. doi: 10.1016/j.apcata.2015.09.026 [26] CHEN Y, LIAO Y F, CHEN L, et al. Performance of transition metal (Cu, Fe and Co) modified SCR catalysts for simultaneous removal of NO and volatile organic compounds (VOCs) from coal-fired power plant flue gas[J]. Fuel, 2021, 289: 119849. doi: 10.1016/j.fuel.2020.119849 [27] YU H, HU W, HE J, et al. Decomposition efficiency and aerosol by-products of toluene, ethyl acetate and acetone using dielectric barrier discharge technique[J]. Chemosphere, 2019, 237: 124439. doi: 10.1016/j.chemosphere.2019.124439 [28] CHEN J Y, LIU J Q, LIU X, et al. Decomposition of toluene with a combined plasma photolysis (CPP) reactor: influence of UV irradiation and byproduct analysis[J]. Plasma Chemistry And Plasma Processing, 2020, 41(1): 409-420. [29] Pan K L, Chang M B. Plasma catalytic oxidation of toluene over double perovskite-type oxide via packed-bed DBD[J]. Environmental Science and Pollution Research, 2019, 26(13): 12948-12962. doi: 10.1007/s11356-019-04714-0 [30] STANISLAV P, JAN M. Temperature-and airflow-related effects of ozone production by surface dielectric barrier discharge in air[J]. European Physical Journal D, 2014, 68(10): 310. doi: 10.1140/epjd/e2014-50393-x [31] BO Z, HAO H, YANG S L, et al. Vertically-oriented graphenes supported Mn3O4 as advanced catalysts in post plasma-catalysis for toluene decomposition[J]. Applied Surface Science, 2018, 436: 570-578. doi: 10.1016/j.apsusc.2017.12.081 -




 下载:
下载: