-
膜法水处理技术因其具有选择性好、适应性强和能耗低等优点而被广泛应用,但膜污染问题伴随着膜技术的应用也随之而来[1-3]。膜污染是在过滤过程中,通过对水中悬浮颗粒物、胶体,溶解性有机物的截留、吸附、沉积作用导致膜渗透性降低的现象,从而造成膜通量衰减,使用寿命降低和运行成本增加,严重影响了膜性能的发挥[4-7]。膜污染是无法避免的,如何能有效的缓解膜污染成为了一个研究热点[8-9]。
近年来,电辅助抗膜污染技术受到了广泛的关注,该技术是在电辅助条件下通过静电排斥、电润湿、电化学氧化等功能降低膜与污染物之间的相互作用来减缓膜污染[10-11]。SUN等[12]报道了通过电化学还原作用产生氢气泡减少污染物附着,通过电化学氧化降解膜表面污染,通过电泳湍流再生膜表面等多种电辅助效应协同抗膜污染,取得了较好的抗膜污染效果。SUN等[13]利用电辅助絮凝不但减缓了膜污染,还增强了膜对有机分子的去除率。WERNER等[14]利用电化学还原产生低浓度双氧水,阻止细菌黏附膜面,从而延缓膜污染。
电辅助抗膜污染技术具有高效、简单、无需外加药剂等优点,为缓解膜污染提供了新的方向。电辅助抗膜污染技术的关键是导电膜,目前导电膜主要采用纳米碳材料或者金属材料制备。制备的导电膜存在着诸多问题:通过单纯纳米碳材料组装制备的导电膜机械强度差,制备工艺复杂[15];通过金属材料制备的导电膜稳定性差,制备工艺复杂制备成本昂贵[16-17]。目前,寻找一种稳定性好、机械强度高、制备工艺简单能大规模生产的导电膜成为了影响电辅助抗膜污染技术应用的关键因素。
本研究通过真空抽滤交联技术制备了具有导电性能的CNT-PVDF复合中空纤维膜,并考察了该复合中空纤维膜的结构形态、稳定性和机械强度,通过调控孔径、电导率等因素对导电复合中空纤维膜的制备工艺进行了优化,且研究了模拟污水和实际污水的电辅助膜过滤抗膜污染的性能,为新型导电膜的发展和抗膜污染研究提供参考。
全文HTML
-
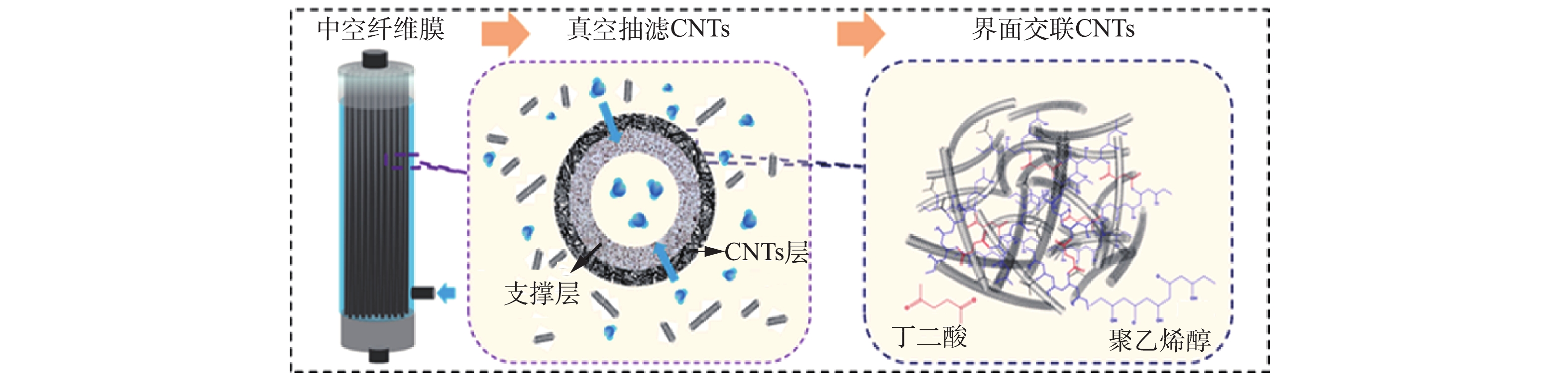
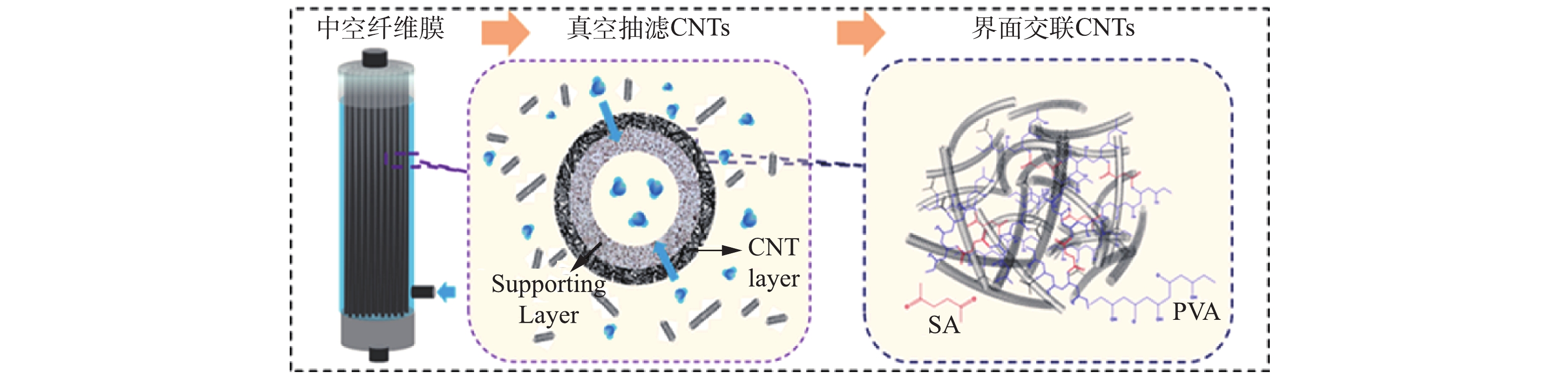
导电CNT-PVDF复合中空纤维膜是在酸性条件下利用酸化CNT表面的羧基与聚乙烯醇表面的羟基发生反应,再由丁二酸这种二元羧酸进一步交联固化,制备出具有稳定CNT导电功能层的复合中空纤维膜。该中空纤维膜的真空抽滤交联制备过程如图1所示,主要包括支撑体商业中空纤维膜预处理和导电CNT-PVDF复合中空纤维膜的制备2个步骤。
将作为支撑体的商业PVDF中空纤维膜用乙醇超声处理10 min,然后利用去离子水将乙醇清洗干净,60 ℃烘干备用,其中商业PVDF膜采用浸涂工艺在尼龙支撑层表面覆盖PVDF制备,平均孔径为400 nm,孔隙率为50~60%,拉伸强度大于2.5 MPa·N−1,断裂伸长率大于160%,爆破压大于0.4 MPa,弯曲因子为4.5~4.8。
配制浓度为0.5 mg·mL−1的CNT分散液。将商业中空纤维膜封装于中空纤维膜反应器中,再将CNT分散液注入反应器,将反应器出口接真空泵抽滤制备CNT层,通过先抽滤一定量的聚乙烯醇(PVA)溶液,再浸泡于丁二酸(SA)溶液中的2步交联反应固定CNT。交联反应完成后,将中空纤维膜取出,用高纯水清洗掉残余的PVA和SA溶液,60 ℃条件下干燥固化得到导电CNT-PVDF复合中空纤维膜。
-
使用扫描电镜(SEM,Hitachi S-4800型,日本Hitachi公司)对导电CNT-PVDF复合中空纤维膜细微结构进行观察。使用数码相机(DSC-WX300型,日本Sony公司)对导电CNT-PVDF复合中空纤维膜表面形态进行观察记录。使用毛细流孔径测试仪(Porolux 1000型,德国Porometer公司)测定膜孔径。使用四探针测试仪(2400型,美国Keithley公司)测定膜导电性。使用X射线光电子能谱分析仪(XPS,ESCALAB 250型,美国Thermo VG公司)对复合中空纤维膜的组成成分进行表征分析。使用电化学工作站(CHI660D型,上海市辰华仪器有限公司)对复合中空纤维膜的电化学性能进行分析。纯水通量测试条件:应用压力为0.1 MPa,测试时间为20 min,通过天平确定出水质量来计算通量。
功能层机械稳定性分别利用超声振荡实验(数字超声清洗机PS-08A型,东莞市洁康超声波清洗机设备有限公司)和纳米划痕实验(划痕试验机CSR-01型,日本RHESCA公司)进行评估。稳定性相关实验是在同样PVDF材质的平板微孔滤膜上进行,以便于后期的结果对比和测量。超声振荡实验是在固定CNT量的条件下,调控不同质量分数(0、0.05%、0.1%、0.2%、0.4%和0.6%)的PVA溶液,用于功能层交联,将成膜称重后,置于超声中超声振荡处理0.5 h,取出彻底干燥后,再次称重,2次重量差值即为CNT损失量。
-
抗污染性能测试是在实验室自制的电辅助膜过滤组件上(图2)进行的。抗污染实验以实验室配制的10 mg·L−1腐殖酸溶液为目标物,在错流比为1∶1,压力为0.05 MPa条件下运行,施加辅助电压为−2~2 V,膜作为工作电极,不锈钢丝网作为对电极。根据膜通量和膜表面形态的变化对膜污染程度进行评估。电辅助系统采用2电极体系,直流稳压电源(WYJ-0~15 V/5A型,上海市山杰电气科技有限公司)为电辅助膜过滤提供槽压。低压膜实验设备(TYLG型,济南博纳生物技术有限公司)为实验提供运行压力和错流动力。同时,本研究针对实际污水也进行了电辅助膜过滤测试。使用的实际污水水样采自大连市某污水处理厂序批式活性污泥工艺(SBR)中SBR反应池出水,水样经过沉淀和PP棉(5 μm孔径)预处理后进行实验,水样水温为22.5 ℃,pH为7.59,电导率为2.27 mS·cm−1。 主要污染物指标浓度如下:COD为92 mg·L−1,UV254为0.62 cm−1,SS为4.8 mg·L−1,总菌数为103 CFU·L−1。实验在错流比为1∶1,压力为0.05 MPa下运行,采用施加辅助电压−2 V和不加电压2种模式运行,膜作为工作电极,不锈钢丝网作为对电极。实际废水实验采用间歇式运行模式,每运行4 h后,利用出水进行反冲洗再生30 min,然后再继续运行。
1.1. 导电CNT-PVDF复合中空纤维膜的制备
1.2. 功能性碳膜的表征
1.3. 导电CNT-PVDF复合中空纤维膜的抗污染测试
-
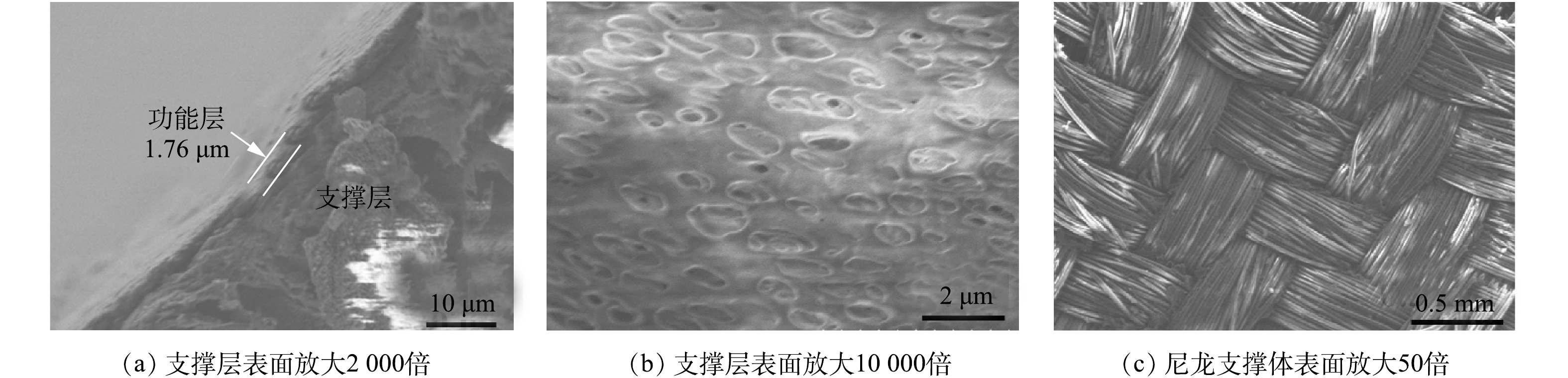
图3为导电复合中空纤维膜电镜图。图3(a)为制备的CNT-PVDF复合中空纤维膜的截面图。可以看出,膜结构分为2层:上层为交联的CNT功能层,CNT负载量为0.5 mg·cm−2时,功能层厚度在2 μm左右;下层为PVDF覆盖在尼龙支撑体上构成的支撑层,尼龙支撑体为网状编织结构(图3(c)),PVDF厚度在5 μm左右。功能层和支撑层连接紧密,未发现有明显的裂痕和破损。 图3(b)为支撑层PVDF表面结构,可以看出,支撑层表面存在大量直径在2 μm左右的大孔,这有利于提高膜渗透性。
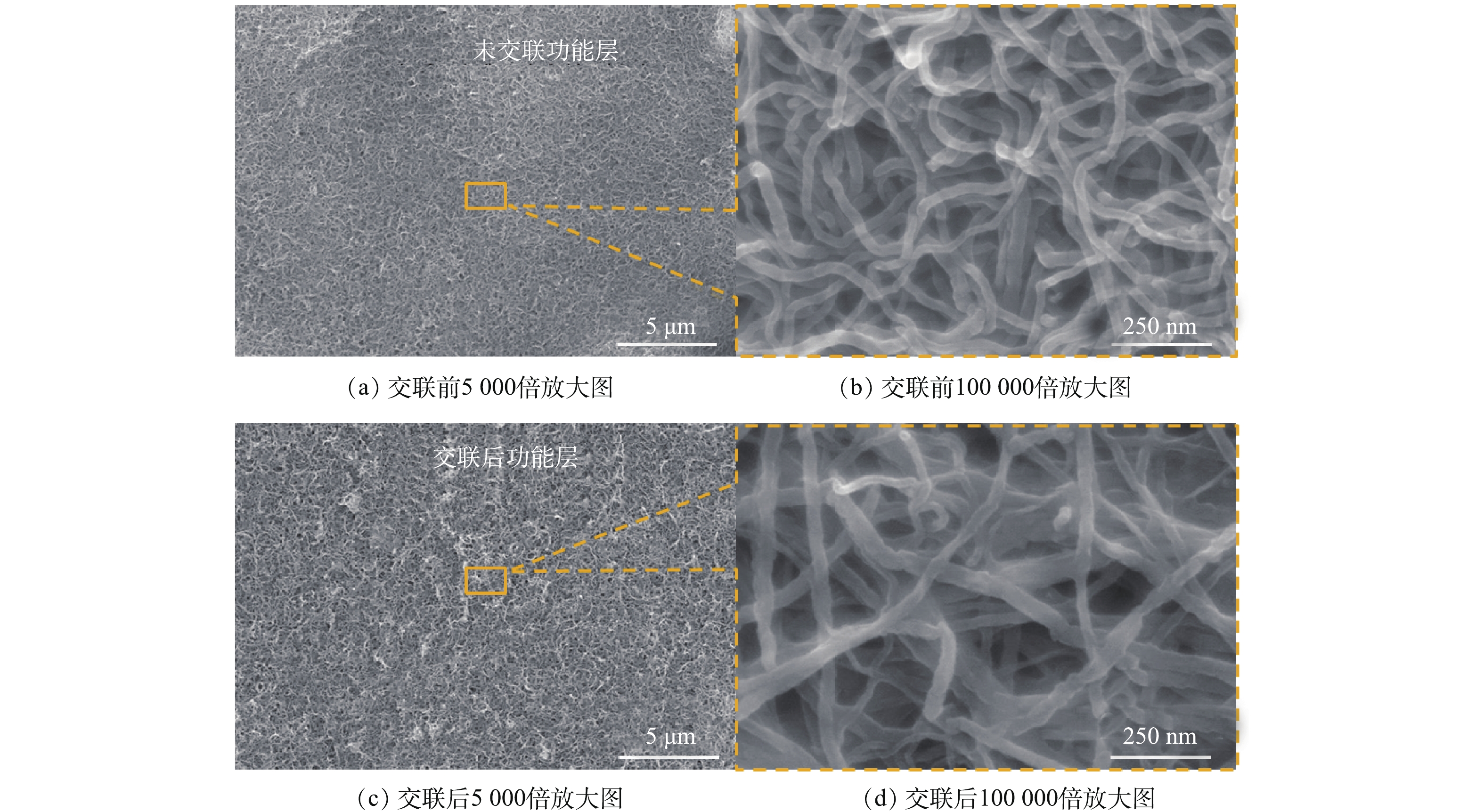
图4展示的是CNT-PVDF复合中空纤维膜CNT功能层在不同状态下的扫描电镜照片。由图4(b)和图4(d)可知,CNT功能层在交联前较为平整,交联后变得较为粗糙。在交联反应后,CNT连接处明显有物质附着。这是因为交联反应的发生过程中大量的聚乙烯醇和丁二酸参与了反应,附着在CNT表面和CNT的连接处,这些交联剂起到了连接CNT的作用,有助于增强功能层整体的结合力。
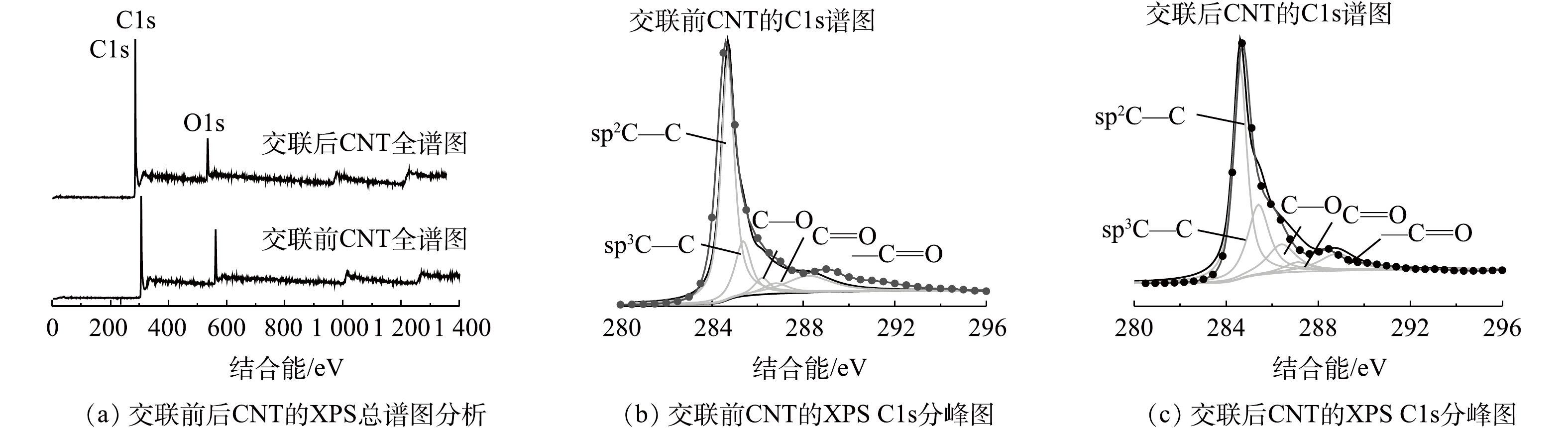
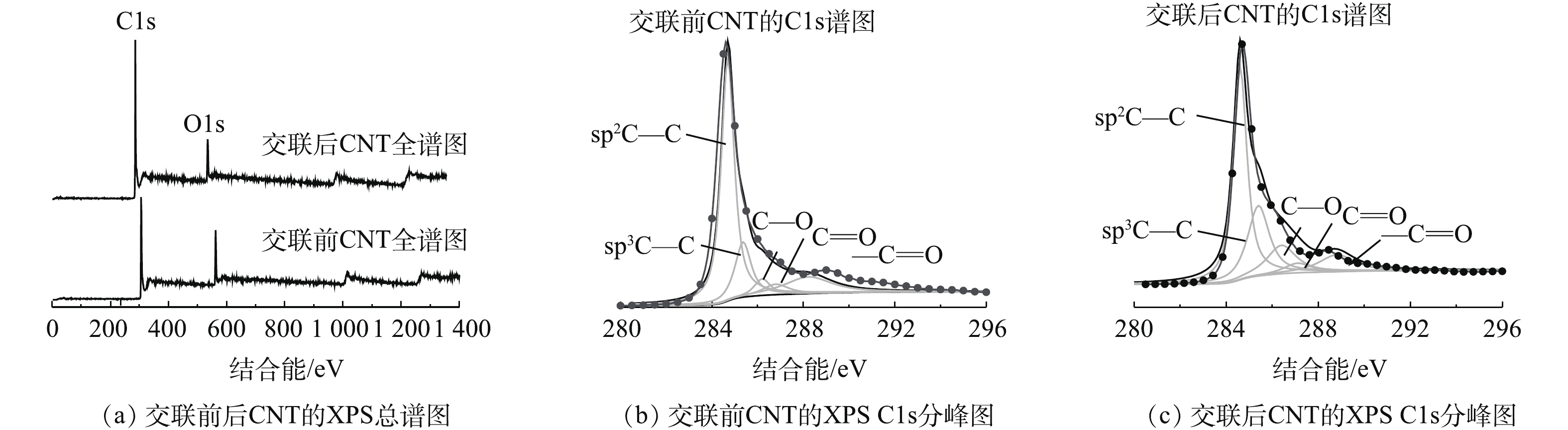
图5是交联反应前后的CNT的XPS谱图。可以看出,碳元素主要以sp2C—C(284.6 eV),sp3C—C(285.4 eV),C—O(286.4 eV),C=O(287.1 eV),—C=O(288.8 eV)的形式存在。而且交联反应后C—O(13.4%)比交联反应前C—O(4.95%)显著增加,这说明随着交联反应的发生,聚乙烯醇交联在CNT表面,聚乙烯醇含有大量未参与反应的C—O基团,增加了交联反应后CNT的C—O基团含量。CNT表面元素含量分析结果表明:交联前CNT中碳含量为86.32%,氧含量为13.68%;而交联后CNT中碳含量为80.69%,氧含量为19.31%。交联反应之后,CNT表面氧元素含量增加也证实了含有较多氧元素的聚乙烯醇和CNT通过共价键结合在一起。
-
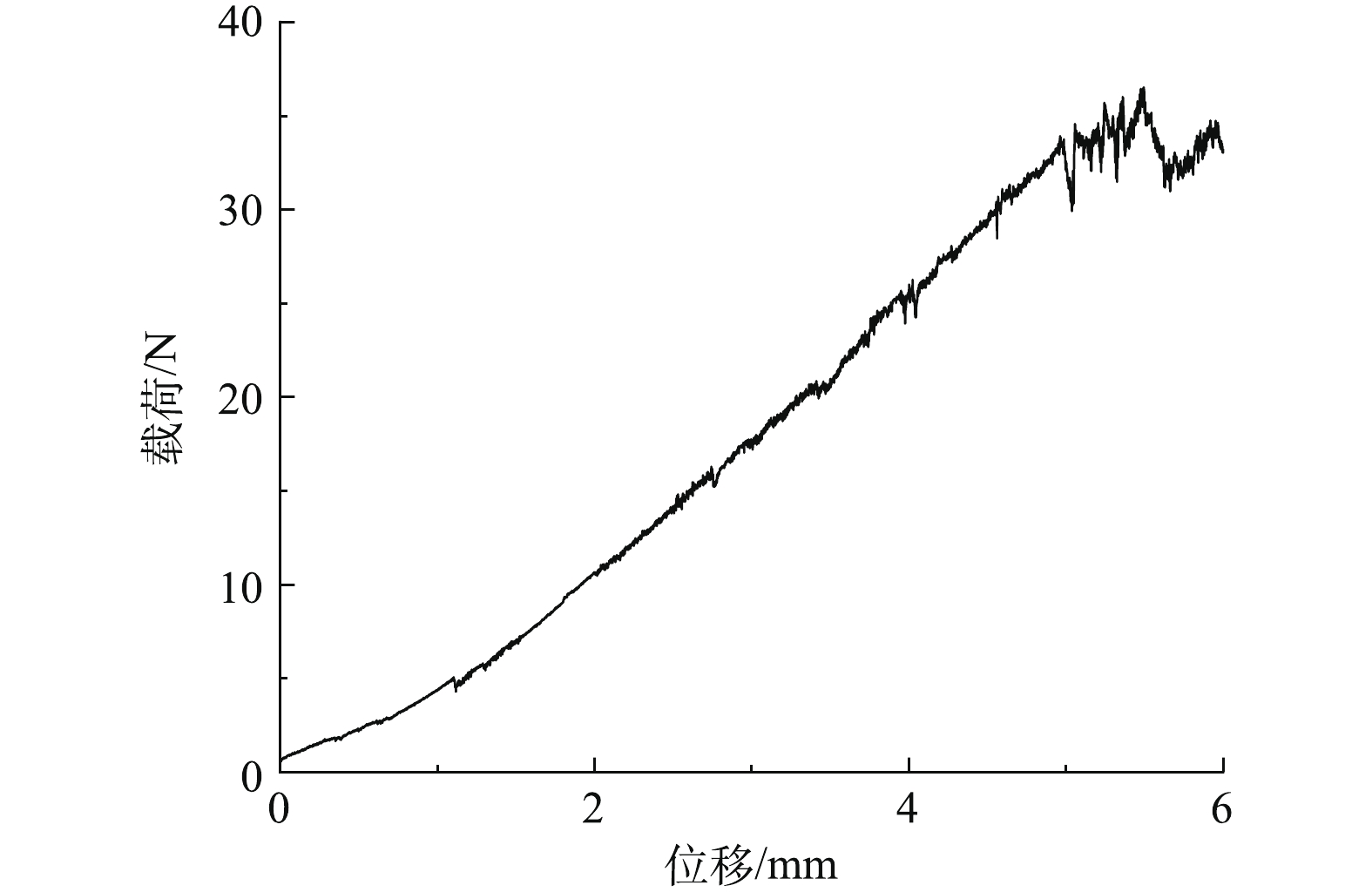
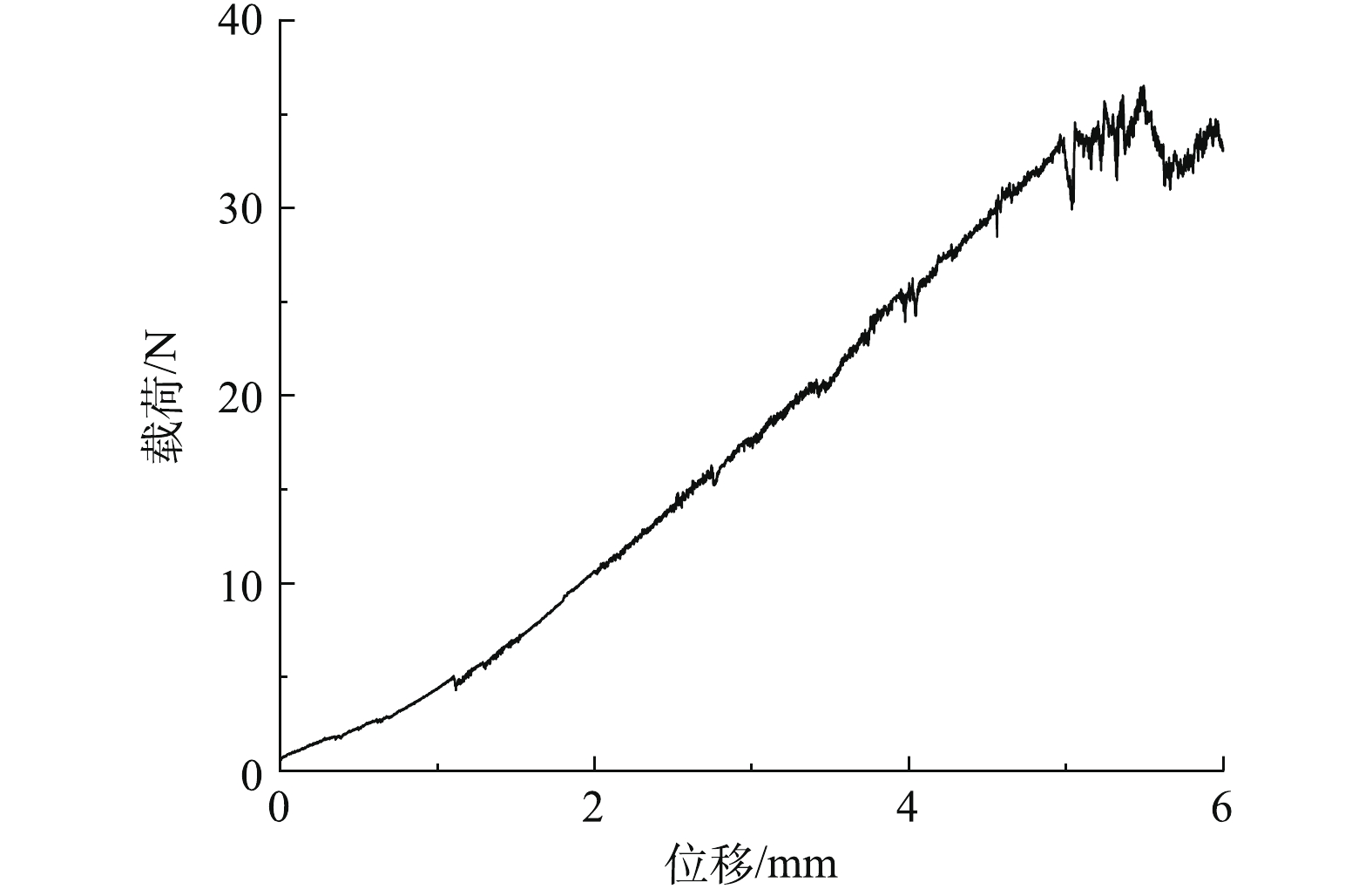
中空纤维膜是自支撑结构的分离膜,所以良好的柔韧性和稳定性才能保证中空纤维膜在制备和应用的过程中能保持较长的使用寿命和稳定的分离性能。复合膜的拉伸载荷与位移关系曲线如图6所示,根据式(1)计算弹性模量。
式中:E为弹性模量,MPa;F为载荷,N;l为试样长度,m;R为试样外径,m;r为试样内径,m;Δl为形变量。
因为支撑层由尼龙加固,复合膜未达到断裂极限就发生非弹性形变,所以根据发生非弹性形变前的载荷(33.70 N)和位移(4.97 mm)计算出复合膜相应的弹性模量约为822 MPa,反映了复合膜具有较好的柔韧性。
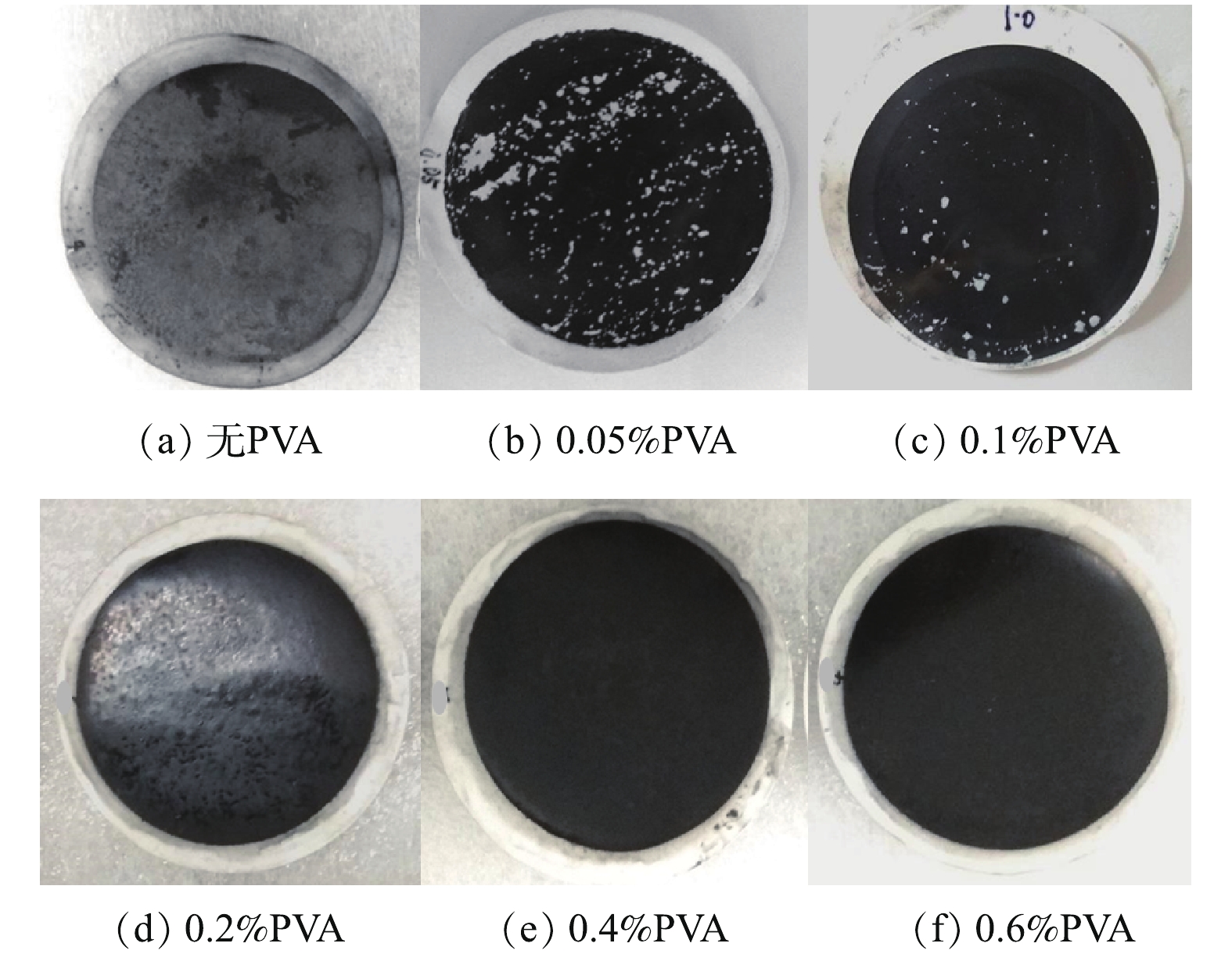
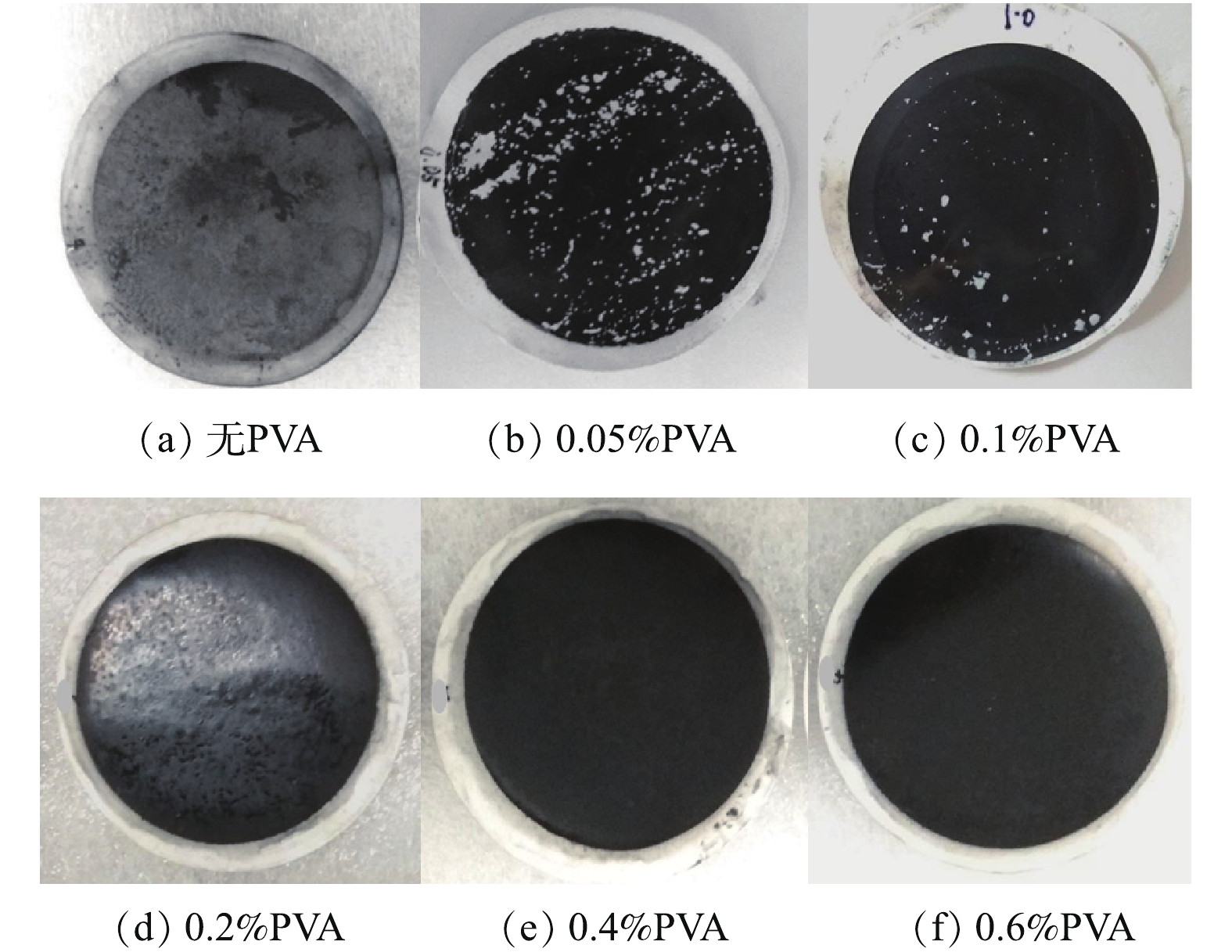
本研究采用不同浓度聚乙烯醇固定导电CNT-PVDF复合中空纤维膜的功能层,并通过物理稳定性测试来筛选出合适的交联剂用量,以保证功能层的稳定性。如图7所示:在0.5 h超声振荡处理以后,未使用交联剂固定的膜表面功能层基本完全脱落,CNT质量损失87.2%;使用质量分数为0.05%和0.1%的聚乙烯醇固定的功能层表面有部分脱落,CNT质量损失分别为26.8%和14.6%;而使用质量分数大于等于0.2%聚乙烯醇固定的功能层表面比较完整,质量损失在1%左右。
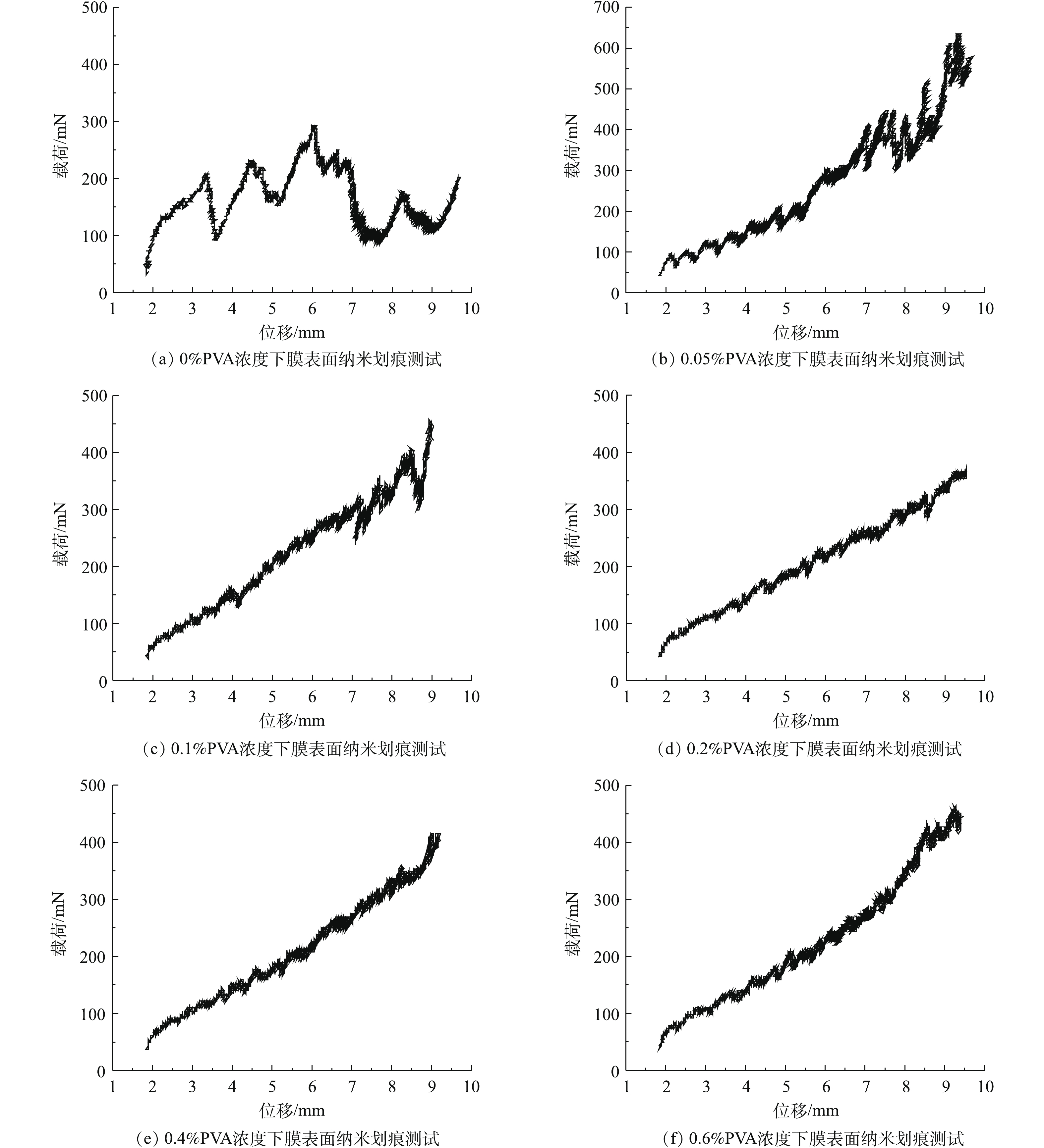
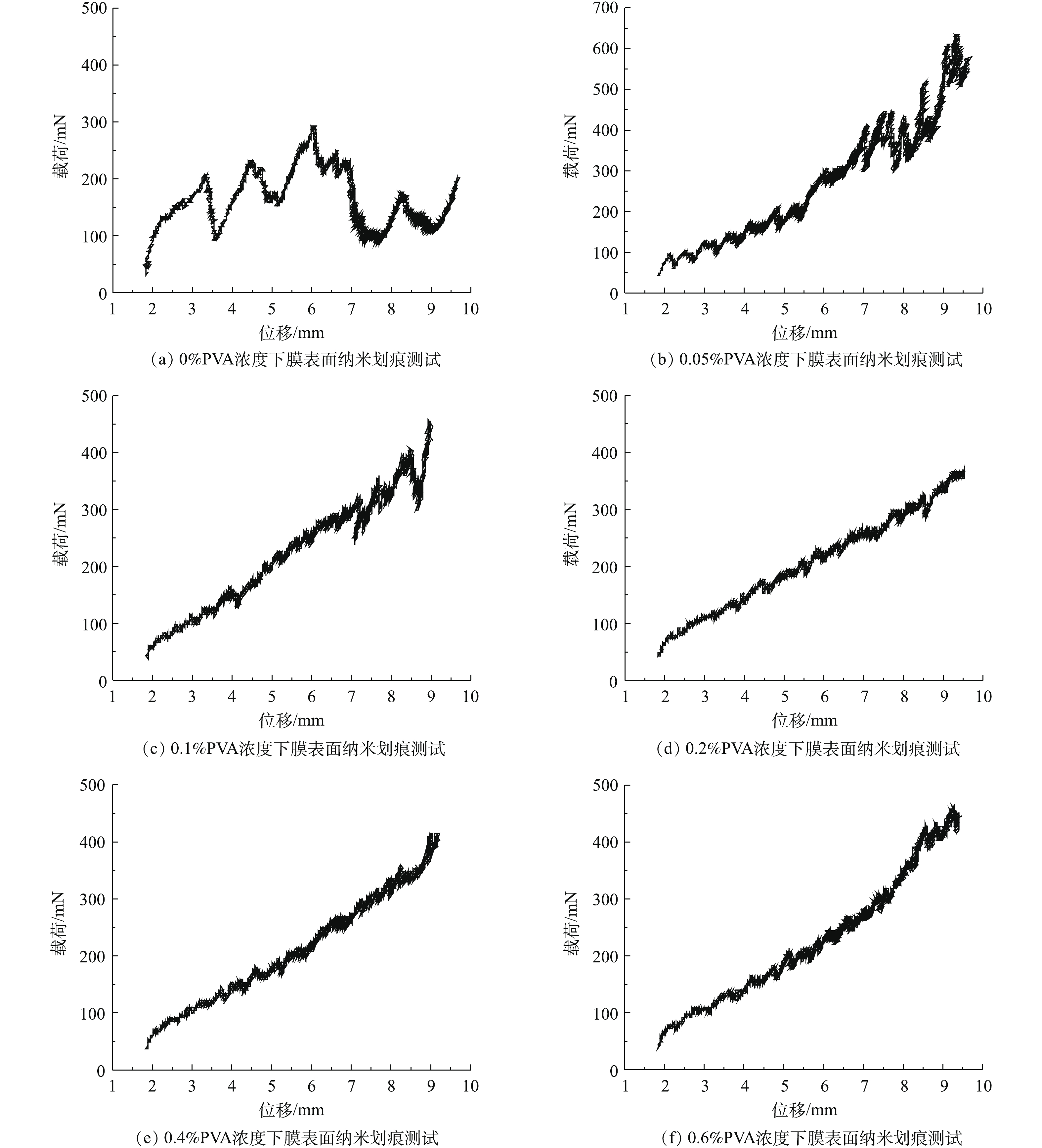
纳米划痕测试结果如图8所示,通过对CNT-PVDF膜表面施加应力进行对比发现:在聚乙烯醇浓度为0、0.05%和0.1%时,膜表面均出现不同程度的破损,发生非弹性变形。在使用的聚乙烯醇浓度≥0.2%时,膜表面发生弹-塑性变形和划痕周围局部断裂,这说明在整个测试过程中的最大载荷未超过功能层与支撑层之间的界面断裂载荷。根据这一现象计算出在聚乙烯醇浓度≥0.2%时,膜表面功能层的界面断裂载荷>400 mN,证明了交联后的CNT-PVDF膜功能层与支撑层之间具有较好的界面稳定性。基于上述结果可确认聚乙烯醇浓度应≥0.2%。
-
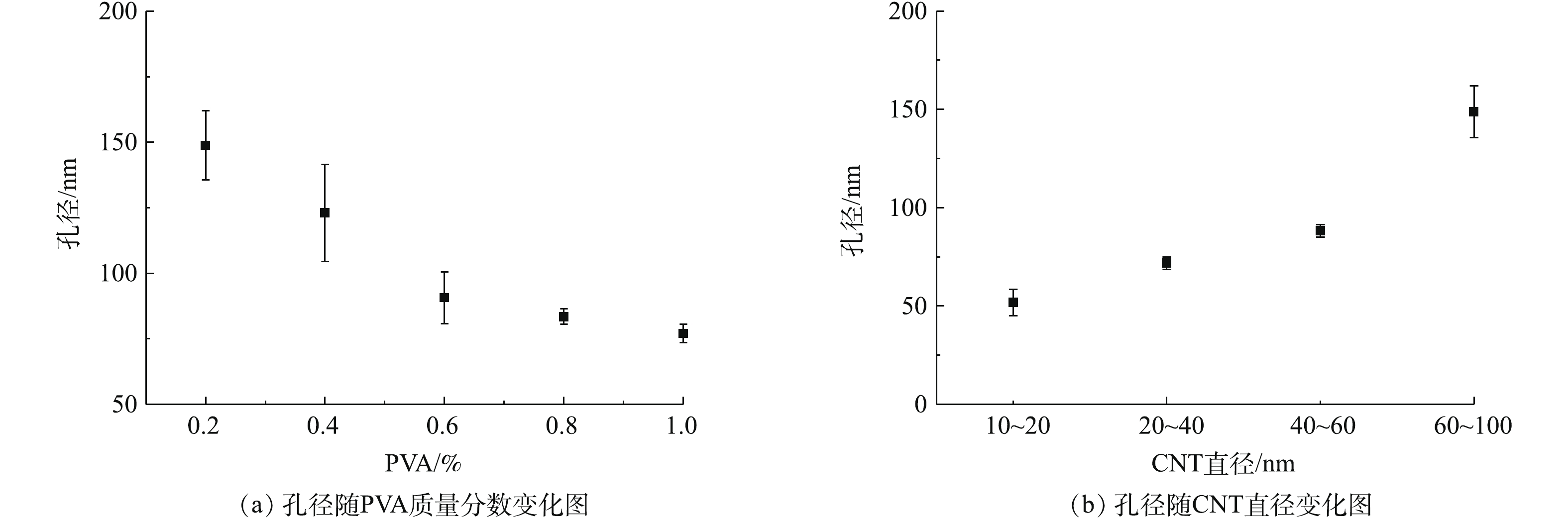
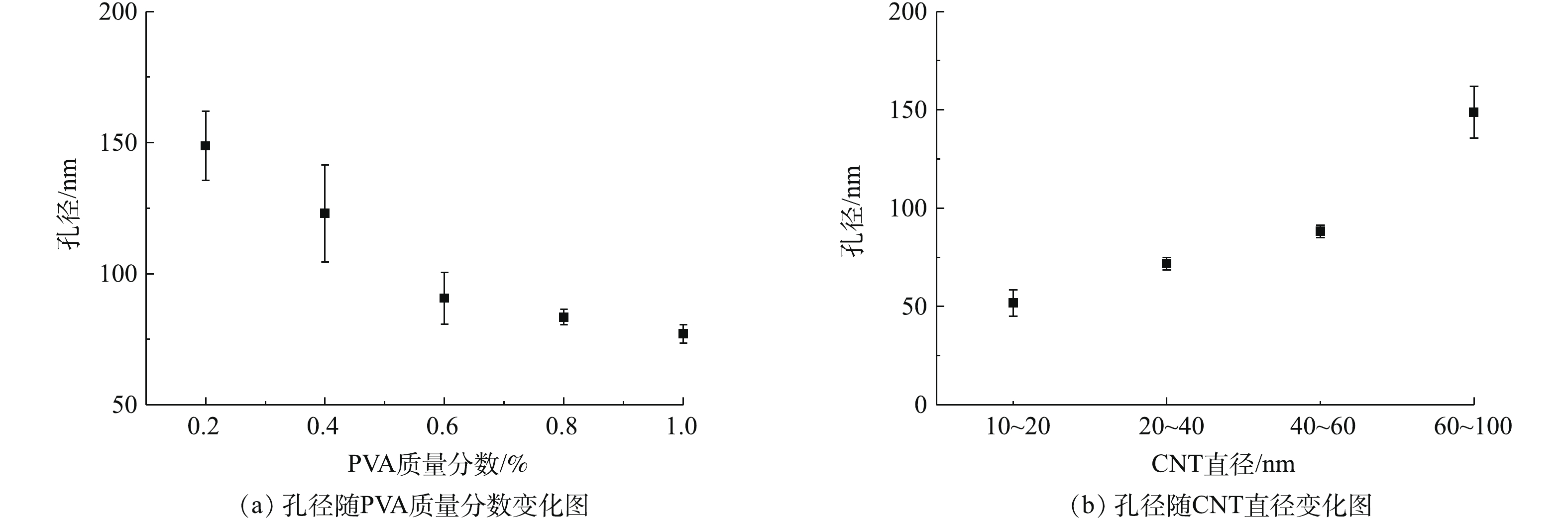
根据制备工艺的特点,聚乙烯醇质量分数和CNT直径可以被用来调控成膜孔径。如图9(a)所示,在使用的CNT直径为60~100 nm时,成膜孔径随着使用的交联剂聚乙烯醇浓度增加,从150 nm左右降至70 nm左右。如图9(b)所示,在使用的聚乙烯醇质量分数浓度为0.2%时,成膜孔径随着使用的CNT直径由50 nm左右增加至150 nm左右。由此可见,通过对聚乙烯醇浓度和CNT直径的调控,能有效调节成膜孔径,且调控范围从微滤到超滤,适用于不同应用需求。
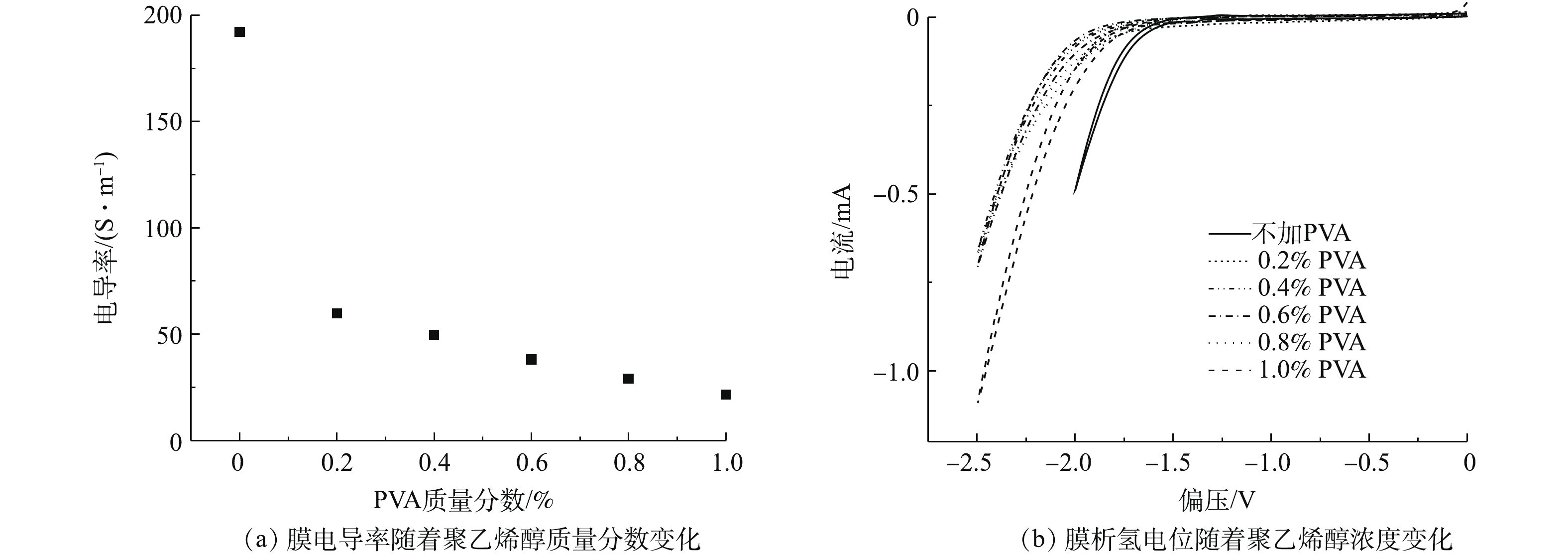
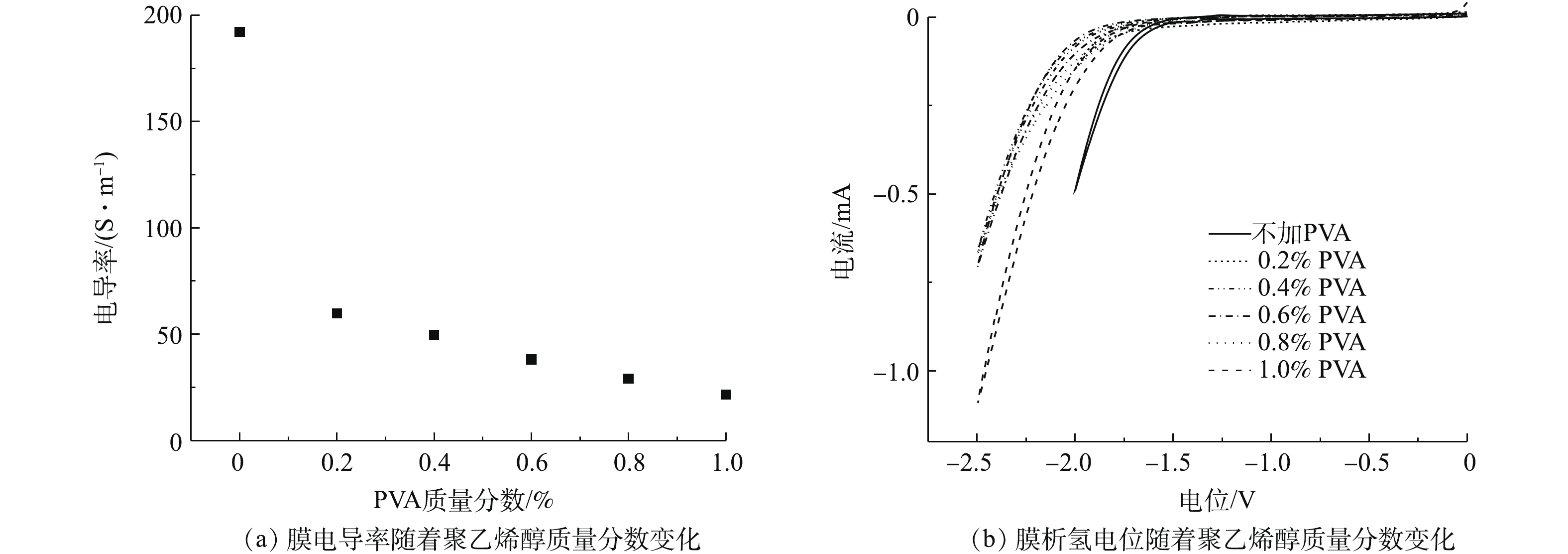
聚乙烯醇的加入也会影响成膜的电导率。如图10(a)所示,根据四探针电阻测试仪的测试结果,膜电导率随着聚乙烯醇质量分数的增加而降低,这是因为聚乙烯醇的存在增大了CNT与CNT之间的距离,带来了功能层整体的电导率降低。且由图10(b)可知, 未交联CNT-PVDF膜的析氢电位(vs Ag/AgCl)为−1.5 V,而聚乙烯醇交联后的CNT-PVDF膜的析氢电位(vs Ag/AgCl)均在(−2.0±0.1) V左右。聚乙烯醇交联后的CNT-PVDF膜具有更低的析氢电位,意味着在不发生电解水反应的条件下,能施加更负电势于交联后的CNT-PVDF膜上,较负电势更有助于电辅助膜过滤功能的实现。
-
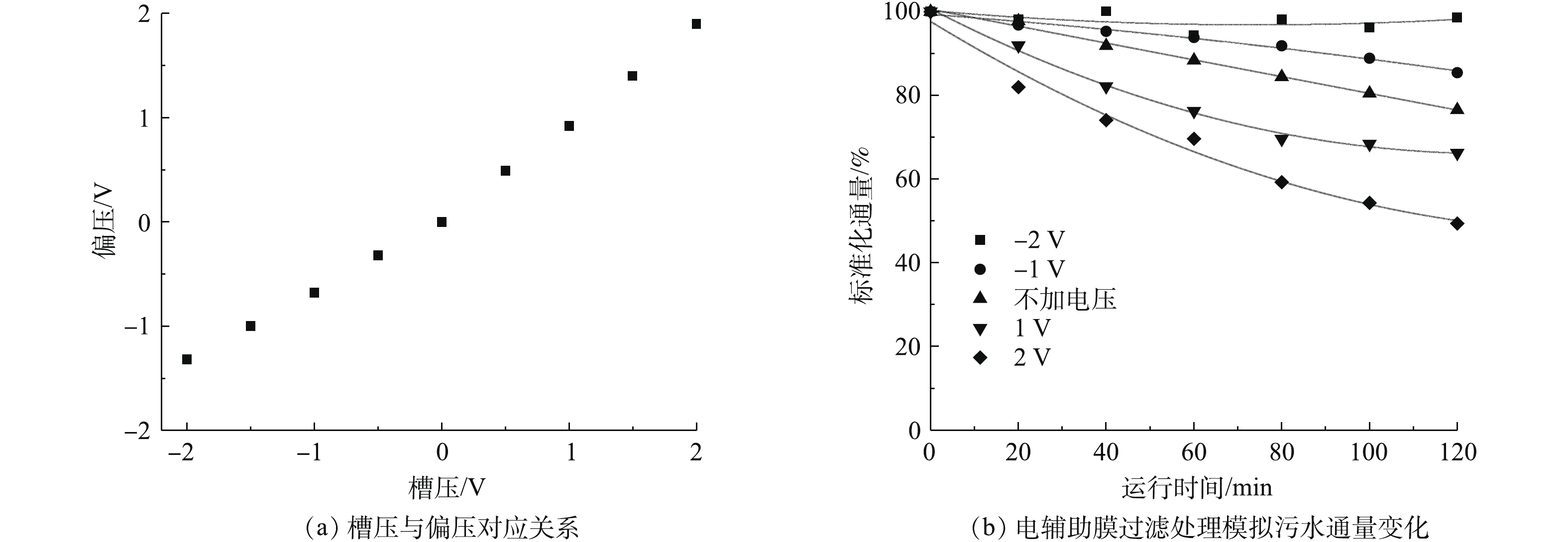
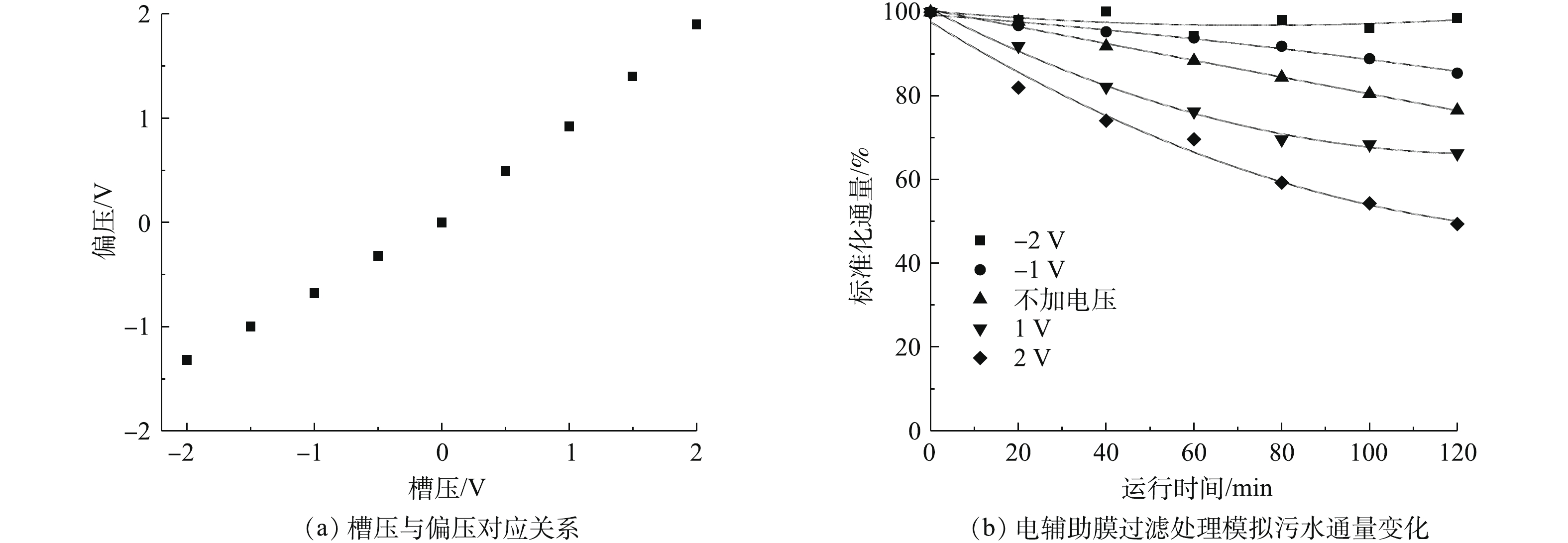
根据以上实验调控结果,选用直径为60~100 nm的CNT,交联剂聚乙烯醇浓度为0.4%,膜平均孔径为(123±18.53) nm,在0.1 MPa压力下,通量为192.8 L·(m2·h)−1的CNT-PVDF中空纤维膜用于水处理。实验中利用10 mg·L−1的腐殖酸溶液来研究导电复合中空纤维膜的电辅助抗膜污染性能。在实验之前,通过电化学工作站测试膜表面的偏压数值。如图11(a)所示,在−2~2 V的应用槽压下,实际施加的偏压处于−1.32~1.9 V。由图11(b)可见,与不施加电辅助膜过滤相比,施加负偏压能减缓膜通量下降速度,负偏压越大,膜污染速率越慢。施加−2 V电压于膜表面,在运行120 min后,膜通量损失在5%以内;不施加电压,运行120 min后,膜通量损失为23.5%。此外,由图11(b)中还可以发现,施加正偏压反而会加快膜通量下降速度,而且正偏压越大,膜污染速率越快。施加2 V电压于膜表面,在运行120 min后,膜通量损失在51.6%左右。出现这一现象的原因是,腐殖酸在pH=7的溶液中呈现出电负性,在负偏压辅助过滤时,膜表面也呈现电负性,同种电性之间的静电排斥作用使腐殖酸不易附着在膜表面形成膜污染,易于在错流模式下,随着水流被冲离膜表面。而在正偏压辅助过滤时,膜表面呈现出正电性,故静电吸引作用使腐殖酸更易在膜表面沉积形成膜污染。所以基于本研究中的CNT-PVDF膜,负偏压条件下电辅助膜过滤能有效降低膜污染速度,而正偏压条件下电辅助膜过滤会加快膜污染速度。
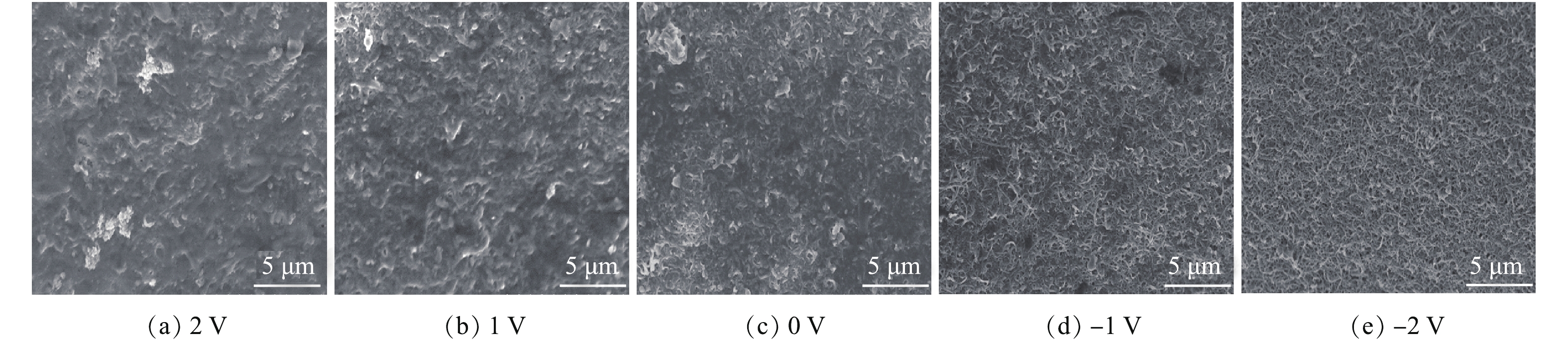
图12为运行120 min后CNT-PVDF膜表面的污染情况。由图12可见:在正电压辅助过滤(膜作为阳极)时,膜表面被一层致密的污染层覆盖,无法观察到功能层CNT的状态;而在不加电时,膜表面大部分区域被污染层覆盖,污染层比正电压辅助过滤时要薄,有部分CNT暴露出来。这一现象验证了在正电压辅助条件下,膜通量衰减速度大于不加电压条件下的衰减速度,正电压辅助加重了膜腐殖酸污染情况。在负电压辅助过滤(膜作为阴极)时,膜表面功能层CNT清晰可见,只有少许位置存在小块膜污染层,这说明了负电压有助于膜腐殖酸污染情况的减轻。
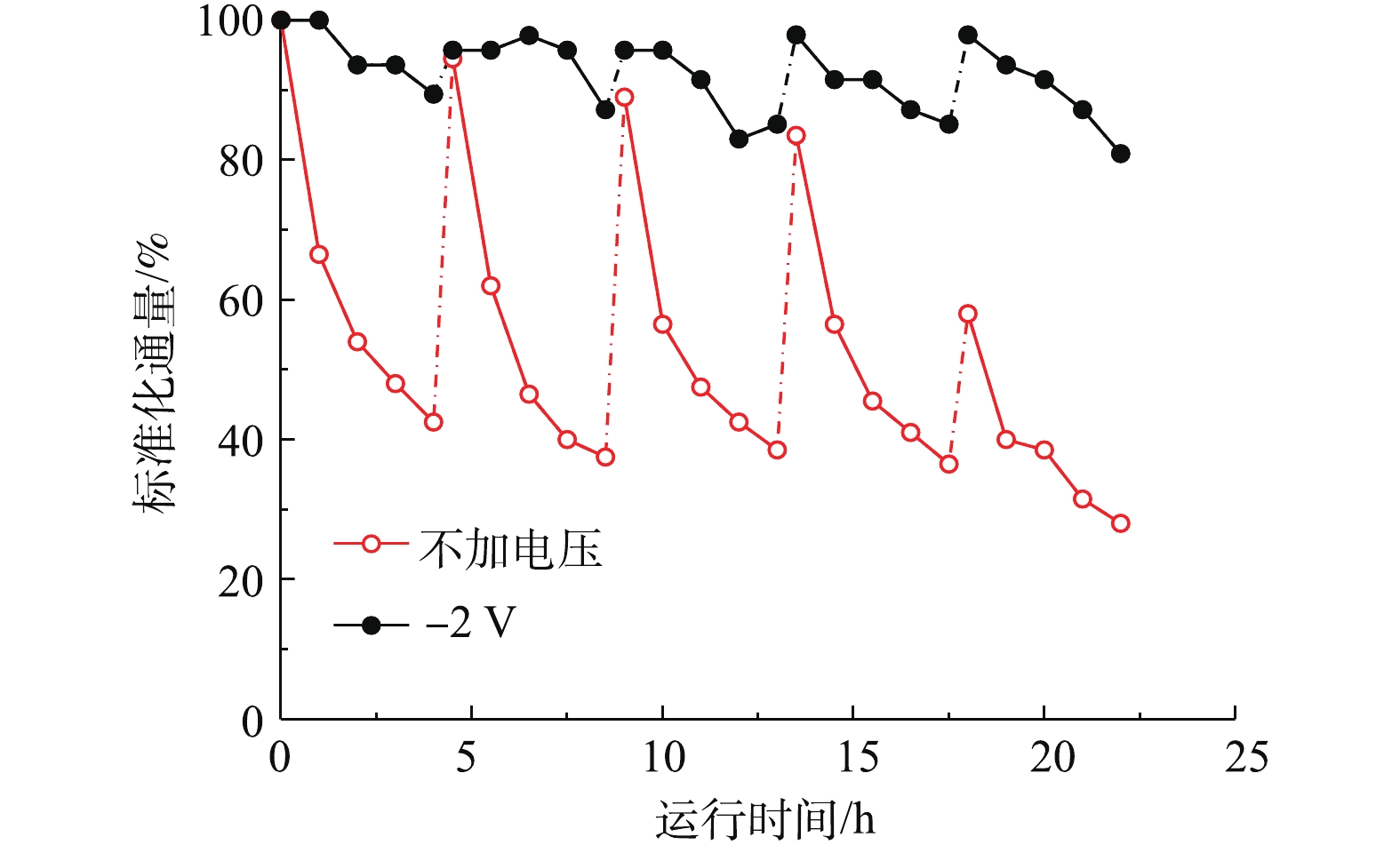
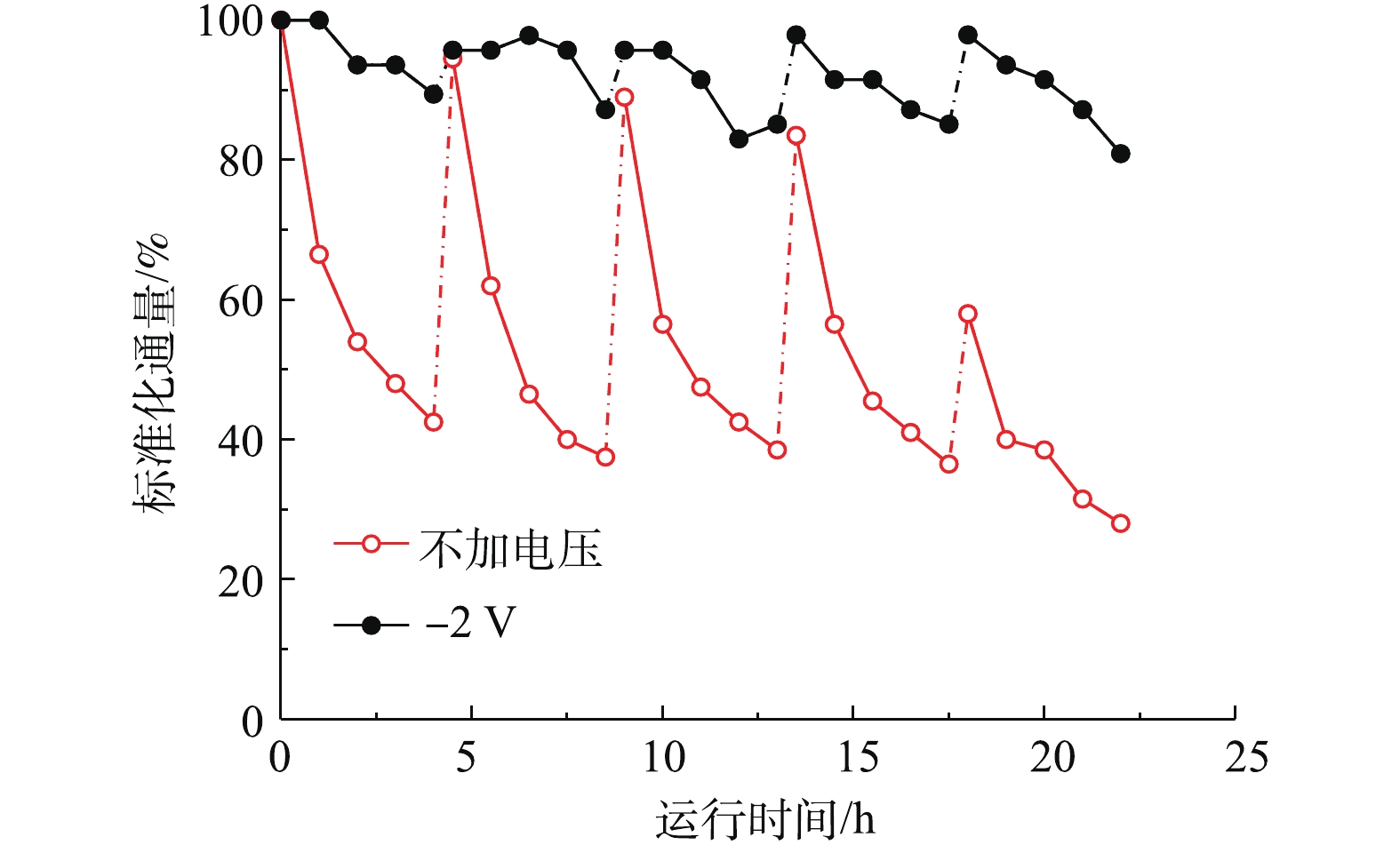
基于上述结果,在电辅助条件下,研究了CNT-PVDF针对实际污水水样的处理效果。如图13所示,在处理实际污水时,在每个运行周期里,−2 V电辅助和开路条件下的膜通量均随着运行时间的增加而逐渐下降,但是−2 V电辅助条件下,膜通量衰减速度远远小于开路状态下的速度。在5个运行周期内,−2 V电辅助过滤通量衰减均小于10%,而单纯过滤过程在5次运行周期后,通量衰减至初始通量的28.0%。在4 h运行结束后,经过0.5 h的反冲洗,−2 V电辅助和开路条件下的膜通量均能得到再生。但是由图13可知,在−2 V电辅助条件下,4次反洗再生后的膜通量均能恢复初始运行通量,而开路状态下,反洗再生后的膜通量随着反洗次数的增加逐渐降低,最终通量恢复为初始通量的58%,这说明不可逆污染已经发生,反洗无法彻底恢复膜初始运行通量。膜污染主要包括不可逆的吸附堵塞污染和可逆的浓差极化形成的凝胶层污染,在运行过程中,这2种膜污染逐渐形成并导致膜渗透性下降,通量衰减。其中可逆污染能在反冲洗过程的水力冲刷下逐渐缓解消除,而不可逆污染却不能有效通过水力冲刷消除。所以随着运行时间的延长,不可逆污染逐渐积累,从而产生明显的渗透性影响。但在电辅助条件下,静电排斥作用和错流过滤水力冲刷作用降低了水中荷负电的主要污染物接触膜面,从而形成膜污染的概率,部分凝胶层污染也能通过反洗消除,所以电辅助减缓了不可逆污染积累的速度,复合膜能通过反冲洗较大程度地恢复通量。通过运行通量的变化可以证明,负偏压−2 V辅助过滤能有效减缓膜污染。
在抗膜污染测试过程中,考察了电辅助膜过滤和单纯膜过滤对实际污水中的COD和总菌数的去除效果。−2 V槽压电辅助膜过滤过程对于COD去除率达到65.2%,出水COD仅为32 mg·L−1;而单纯膜过滤过程对于COD去除率为56.5%,出水COD为40 mg·L−1。在电辅助作用下,膜表面与水中因为吸附作用带负电的溶解性有机物和胶体产生静电排斥作用,降低了其渗透过膜或者通过吸附扩散过膜的概率,提高了对COD的去除率。在−2 V槽压下,电辅助膜过滤和单纯膜过滤过程均对污水中总菌数达到100%的去除率,这是因为膜孔径为106 nm,较菌体尺寸((0.3~2) μm)小,故菌体通过尺寸筛分作用可被膜完全截留。
2.1. 导电复合中空纤维膜的形态结构及组成分析
2.2. 导电CNT-PVDF复合中空纤维膜的柔韧性和功能层稳定性分析
2.3. 导电复合中空纤维膜的参数调控
2.4. 导电复合中空纤维膜的电辅助抗污染分析
-
1)导电CNT-PVDF复合中空纤维膜的孔径尺寸和电导率随着交联剂聚乙烯醇浓度的升高而降低,孔径尺寸还随着使用CNT直径的增加而增大。当CNT负载量为0.5 mg·cm−2,PVA浓度为0.4%,采用CNT直径为60~100 nm时,膜功能层厚度在2 μm左右,膜平均孔径为(123±18.53) nm,在0.1 MPa压力下,纯水通量为192.8 L·(m2·h)−1。
2)通过酸化CNT表面羧基与聚乙烯醇的羟基发生交联反应以固定CNT,该技术增强了导电功能层的稳定性。在交联剂聚乙烯醇质量浓度≥0.2%时,CNT功能层在0.5 h的超声振荡测试中能保持完整,说明该复合中空纤维膜具有较高的物理稳定性。
3)在CNT-PVDF复合中空纤维膜上施加−2 V槽压时,采用电辅助膜过滤处理实际污水,静电排斥作用可以有效减缓膜通量衰减速度,降低膜污染程度,5个运行周期内的膜通量衰减均小于10%,反冲洗再生时能完全恢复膜初始通量。而单纯膜过滤在5个运行周期内的膜通量衰减了72%,反冲洗再生后膜通量不能有效恢复。在电辅助条件下,该复合中空纤维膜表现出优秀的抗膜污染性能。



 下载:
下载: