双金属系统还原脱溴降解多溴联苯醚(PBDEs)研究进展
Progress on bimetallic system debrominated reduction technology for degradation of polybrominated diphenyl ethers
-
摘要: 多溴联苯醚(PBDEs)是一类新型持久性有机污染物。双金属系统催化还原脱溴技术作为一种处理环境中PBDEs的方法,具有出较好的应用前景。综述了双金属系统催化还原PBDEs脱溴的研究进展,详细阐述了双金属系统催化PBDEs脱溴降解的机理、途径以及不同双金属系统的脱溴效能。提出了利用双金属系统降解PBDEs存在的问题和发展前景。Abstract: Polybrominated diphenyl ethers (PBDEs) is a new class of persistent organic pollutants. Bimetallic system debrominated reduction technology is a novel method to efficiently treat PBDEs in the environment. The progress in research on the degradation of PBDEs, the paths, and the mechanism of the degradation reaction by a bimetallic system are addressed in this study and the dehalogenation performance by different bimetallic systems are discussed. In addition, certain problems and prospective outlooks on developing a bimetallic system technology for PBDEs degradation are covered.
-
随着产业升级和城市扩张,大量工厂搬迁或废弃后遗留的场地存在土壤污染问题,需要进行土壤修复才能再次开发[1]。电阻加热技术具有对环境扰动小,受土壤异质性影响小,处理深度大等优点,尤其适合修复含有挥发性、半挥发性有机污染物的污染场地[2-5]。但在实际修复工程中,ERH技术的工程参数设计,例如电极间距、电场强度等,都会显著地影响场地电阻加热的实际效果[6],导致修复周期和成本控制的不确定性。修复场地的污染物分布、地下水流场和土壤特性等往往差异较大,但工程师只能根据已有工程经验和有限的取样勘探结果,进行原位加热工程的参数设计。若能够采用建模的方法,对场地条件下的加热过程进行预测,将有利于减少设计的盲目性,帮助缩短工程周期和控制修复成本。
目前,对于ERH技术的数值模型已经有了一定的探索和应用。HIEBERT等[7-8]开发了用于模拟单相电阻加热过程的二维有限差分模型,并研究了不同的横卧电极设置方式对非均质含油地层的加热效果的影响。CARRIGAN等[9]将改进的欧姆加热模型与非等温多孔流动和传输模型进行了耦合,研究了电极阵列的电相位如何影响电阻加热的均匀性。MCGEE等[10]进一步简化了模拟多相电阻加热的欧姆方程,并模拟了电阻加热从非均质油砂中回收沥青的过程。KROL等[11]考虑温度对密度、粘度、扩散系数的影响,建立了二维有限差分模型,模拟了电阻加热到50 ℃的情况下对地下水流动的影响,发现地下水流动方向和流速发生显著变化。许丹芸等[12]使用有限元方法模拟了电阻加热土壤过程。
尽管关于ERH技术的数值模型研究已有一定的开展,但一方面,以往的模型对电阻加热土壤过程中的水分蒸发缺乏关注和进一步的验证;另一方面,大部分模型是针对实际场地的验证评估,对如何运用模型指导ERH工艺参数的选取探讨不足。本研究使用COMSOL多物理场耦合软件,基于有限元计算方法开展原位电阻加热温度场模拟研究,建立了考虑土壤水分蒸发的模拟原位电阻加热温度场的数值模型。通过对比土柱装置小试实验和数值模拟的结果,验证了数值模型的准确性,并利用数值模型分析了场地尺度下电场强度、电极间距和地下水流动对电阻加热温度场的影响。本研究结果有助于预测修复周期和优化电极井布设,从而达到节约能源和降低修复成本的目的。
1. 数值模型与实验方法
1.1 控制方程及条件假设
本研究利用COMSOL的传热模块、电流模块、PDE模块以及电流和传热耦合的电磁热模块,构建土壤电阻加热模型。为简化模型概念,在模拟时做如下假设:1)忽略土壤和水在加热过程中密度、热容随温度的变化;2)将土体视为均质且各向同性的多孔介质,土壤初始温度均匀一致;3)忽略土体在加热过程中的热变形;4)忽略电极和土壤之间的接触电阻;5)由于实验土柱较短,需要考虑水分在低于沸点时的挥发[13],但在模拟大尺度的场地加热中,忽略水分的挥发;6)忽略水蒸气对热量传递的影响。
电阻加热土壤过程中能量的控制方程如式(1)所示。
stringUtils.convertMath(!{formula.content}) (1) 式中:ρeff为单元的有效密度,kg·m−3;Ceff为单元的有效热容,J·(kg·K)−1;T为温度,K;t为时间,s;λ为导热系数,W·(m·K)−1;σ为土壤电导率,S·m−1;E为电势梯度,V·m−1;mLG为水的气化速率,kg·(m³·s)−1;ΔHvap为水的潜热,J·kg−1;ρf为流体密度,kg·m−3;Cf为流体热容,J·(kg·K)−1;uf为流体流速,m·s−1。
土壤单元的有效密度ρeff和有效热容Ceff由土壤中固,液,气三相的体积分数决定,如式(2)~式(3)所示。
stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) 式中:θ表示各相的体积分数;ρ为各相的密度,kg·m−3;C为各相的热容,J·(kg·K)−1;下标S,L,G表示固,液,气三相。
土壤含水量的控制方程如式(5)所示。
stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) 式中:DL为导水系数,m2·s−1;α为比例常数,m2·s−1;θL*为残余饱和度。
液态水变为气态水的情况可分为2种,一种是低于水的沸点时的挥发,一种是到达沸点时的沸腾,用式(6)可以得到水的气化速率。需要注意,只有水的饱和蒸气压(p*)大于等于外部气压(pG)且含水量大于0时,沸腾才会发生。可以通过安托因方程[14](式(7))计算不同温度下水的饱和蒸气压,进而判断温度是否到达沸点。
stringUtils.convertMath(!{formula.content}) (6) stringUtils.convertMath(!{formula.content}) (7) 式中:A、B、C为经验常数;mvap为挥发速率,kg·(m³·s)−1;kvap为蒸发速率常数,s−1。
土壤单元的热导率会随着温度和含水量的变化发生极大的变化,在此使用TARNAWSKI等[15]推导出的经验公式,如式(8)~式(9)所示。
stringUtils.convertMath(!{formula.content}) (8) stringUtils.convertMath(!{formula.content}) (9) 式中:下标sat和dry分别表示饱和和干燥状态的土壤;a~g为经验常数;Sw为水饱和度。
电阻加热一般使用低频率电压(50~60 Hz),产生的电磁波长远大于系统的物理尺寸,位移电流可以忽略,因此可以假设电阻加热产生的电场为准静态电场,可以通过将欧姆定律代入电流连续性方程中来求解电势分布,电流连续性方程如公式(10)所示。
stringUtils.convertMath(!{formula.content}) (10) 土壤电导率则使用Archie定律进行计算[16-17],考虑温度对电导率的影响[18],如式(11)所示。
stringUtils.convertMath(!{formula.content}) (11) 式中: ψ为电势,V;φ为孔隙度;m、n和β为经验常数(m为胶结系数,n为饱和度系数,β为温度系数);σL为土壤溶液电导率,S·m−1。
模拟单相交流电加热时,其电势分布与直流电基本一致,可以设置2个电极分别为接地和施加的电压。但模拟三相交流电时,电极电势与直流电则完全不同,三相交流电的电极电势可以视为由虚部和实部组成[19],如式(12)所示。
stringUtils.convertMath(!{formula.content}) (12) 式中:ω为角频率,rad·s−1;t为时间,s;θ为相角,°(三相交流电分别为0 °、120 °和240 °);j为虚部;E0为正弦交流电振幅的绝对值,一般为电压的21/2倍,V。
1.2 几何模型和网格划分
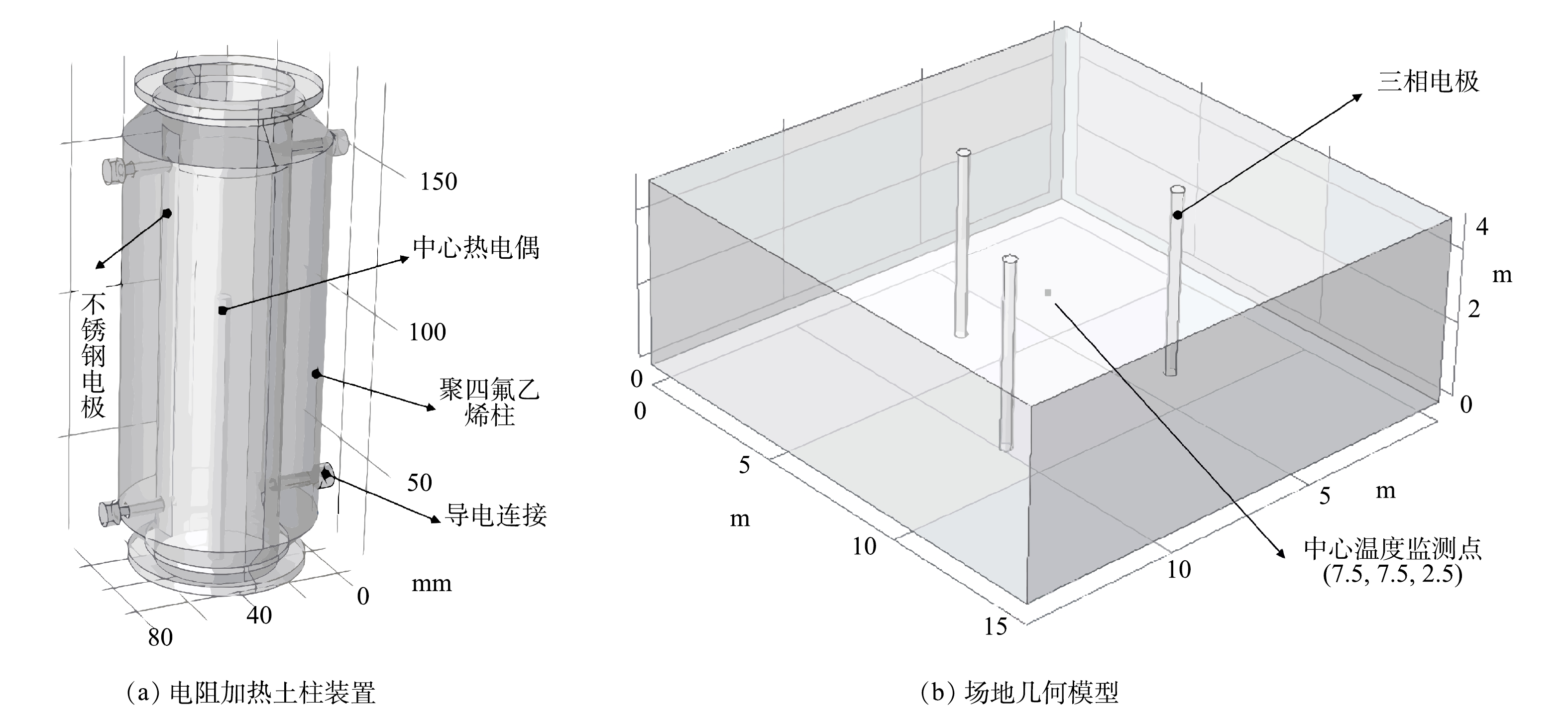
电阻加热土柱装置模型如图1(a)所示,装置高16 cm,内径4.5 cm。装置的罐体用不导电的聚四氟乙烯制成,内部装填细砂。装置2边为对称分布的用螺丝与螺母固定的不锈钢片电极,螺丝作为导电连接,电线连接到螺丝上以引入电压,热电偶从装置底部插入,用于监测土柱中心点的温度。使用Solidworks对电阻加热土柱装置进行建模,得到电阻加热土柱装置的几何模型,再导入到COMSOL中用于模拟,几何模型的网格划分均采用三角单元网格,单元大小选择细化。
为了模拟实际场地中电阻加热的过程,构建了图1(b)所示的三相电阻加热场地模型。场地模型为长宽15 m、高5 m的长方体,在场地中部按等边三角形放置3根半径20 cm、高5 m的电极,电极间距为6 m,并在3个电极构成的等边三角形中心点设置了温度监测点。
1.3 边界条件和参数设置
电阻加热土柱装置数值模拟的边界条件为:1)流动边界条件,所有边界均设置为0通量边界条件,这是因为已在方程中添加了描述水蒸发的汇项;2)温度边界条件,所有边界均设置为自然对流热通量边界;3)电势边界条件,2个电极分别设置为接地和电势,其他边界设置为电绝缘边界。土壤的初始温度、含水量、水的电导率和电极电压根据实测得到的初始值设置。对于各项参数的取值如表1所示。
表 1 数值模拟参数Table 1. Numerical simulation parameters模拟参数 取值 模拟参数 取值 液体密度,ρL 1 000 kg·m−3 温度系数,β 0.02 固体密度,ρS 2 650 kg·m−3 比例常数,α 5×10−6 m2·s−1 气体密度,ρG 1.9 kg·m−3 经验常数,A 8.07131 液体热容,CL 4 200 J·(kg·K)−1 经验常数,B 1 730.63 固体热容,CS 920 J·(kg·K)−1 经验常数,C 233.426 气体热容,CG 1 000 J·(kg·K)−11 胶结系数,m 1.44 湿导热系数,λsat 1.87 W·(m·K)−1 饱和度系数,n 2 干导热系数,λdry 0.23 W·(m·K)−1 水的潜热,ΔHvap 2 257.2 kJ·kg−1 孔隙度,φ 0.5 蒸发速率系数,kvap 1×10−6 s−1 电阻加热场地模拟的边界条件为:1)流动边界条件,模拟地下水位上涨时,底部边界设置为通量边界条件,其他边界设置为0通量边界条件;2)温度边界条件,地下水流入的边界设置为流入边界,流入温度10 ℃,其他边界设置为热绝缘边界;3)电势边界条件,3个电极分别设置为三相电势中的1相,其他边界设置为电绝缘边界。各项参数的设置与土柱装置实验相同。
1.4 实验条件
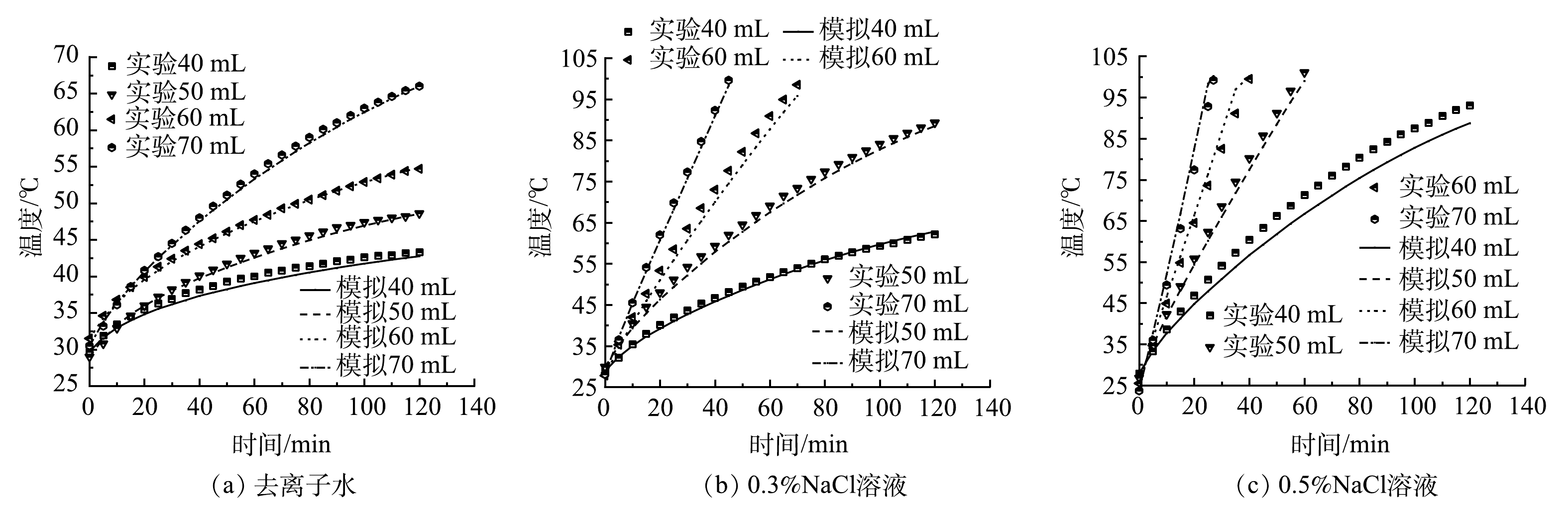
为验证模型在不同的土壤含水量和土壤溶液电导率时的准确性,使用电阻加热土柱装置进行了验证实验。由于装置较小,电场强度较大,故使用电导率较小的细砂充当模拟土壤。分别向300 g细砂中加入40、50、60、70 mL的去离子水,质量分数为0.3%的NaCl溶液和质量分数为0.5%的NaCl溶液,充分搅拌,并装填进装置中。为了保持砂的孔隙度一致,在装填时保证装填高度为15 cm。加入去离子水的砂在100 V的条件下进行电阻加热,加入NaCl溶液的砂在50 V的条件下进行电阻加热。由于模拟只探究电阻加热的升温过程,所以实验只进行到温度到达100 ℃就停止,未加热到100 ℃时则在加热2 h后停止。
为模拟实际工程环境下土壤内热量传递规律,用实际场地模型探究了地下水流动、电场强度和电极间距的变化对土壤温度变化的影响。模拟的工况如表2所示,其他参数与表1一致,土壤溶液电导率设为0.1 dS·m−1。
表 2 数值模拟工况Table 2. Numerical simulation conditions工况 电场强度/ (V·m−1) 初始水饱和度 地下水流速/ (m·s1) 电极间距/m 地下水涨速/ (m·d1) A-1 30 0.6 0 6 0 A-2 60 0.6 0 6 0 A-3 90 0.6 0 6 0 B-1 90/60 0.6 0 3 0 B-2 90/60 0.6 0 6 0 B-3 90/60 0.6 0 9 0 C-1 90 1 0.1 6 0 C-2 90 1 0.2 6 0 C-3 90 1 0.3 6 0 D-1 90 0.6 0 6 0.05 D-2 90 0.6 0 6 0.1 D-3 90 0.6 0 6 0.2 2. 结果与讨论
2.1 电阻加热土柱装置实验及模型验证
图2给出了不同含水率和电导率的情况下,土柱中心热电偶监测的温度变化。可以看出,随着含水量和土壤溶液电导率的增加,中心点加热到100 ℃所需要的时间不断减小。而当含水量较小或者土壤溶液电导率较小时,升温速率较小,甚至出现温度平台的情况。升温速率较小是由于此时土壤电导率较小[20],电流产生的焦耳热较小。升温速率减小则是因为,土柱中挥发掉的水份随着加热时间的增长逐渐变多[21],进一步降低了土壤电导率,使电流产生的焦耳热进一步减少。此外,由图2(a)可以看到,加入不同体积的去离子水后的细砂依然可以被加热,而去离子水的电导率几乎可以忽略不计。这说明,细砂中的离子溶解进入了去离子水中,提高了去离子水的电导率。
为验证模型的可靠性,将不同含水量和土壤溶液电导率的电阻加热实测值和模拟值进行了对比。利用均方误差MSE和平均相对误差MRE评价模拟值和实测值的差异(式(12)~式(13))。
stringUtils.convertMath(!{formula.content}) (13) stringUtils.convertMath(!{formula.content}) (14) 式中:n为实测数据个数;Mi、Si分别为第i个实测和模拟得到的数据。
图2(a)中给出的模拟值趋势线与实测值差异较小,实测值和模拟值均方误差为0.05~0.66,平均相对误差为0.42%~1.97%。较小的均方误差和平均相对误差表明,实测数据与模拟数据之间的偏差较小,模型具有较好的准确性。加入不同体积的0.3%NaCl(图2(b))和0.5%NaCl(图2(c))溶液,土壤溶液电导率随着NaCl溶液的体积和质量分数的增加而增大。对比实测和模拟预测结果,均方误差为0.38~12.29,平均相对误差为1.15%~5.32%。这表明,在较宽的土壤电导率范围内(加入70 mL的0.5%NaCl溶液时,土壤的电导率为1.1 dS·m−1),实测数据与模拟数据之间的偏差也较小,模型用于预测不同电导率的土壤加热过程是可靠的。误差产生的原因可能是,对土壤原有的电导率,实验过程中空气热对流导致的热量散失以及水分挥发速率的估值存在一定的偏差。
2.2 场地条件下电场强度和电极间距对电阻加热的影响
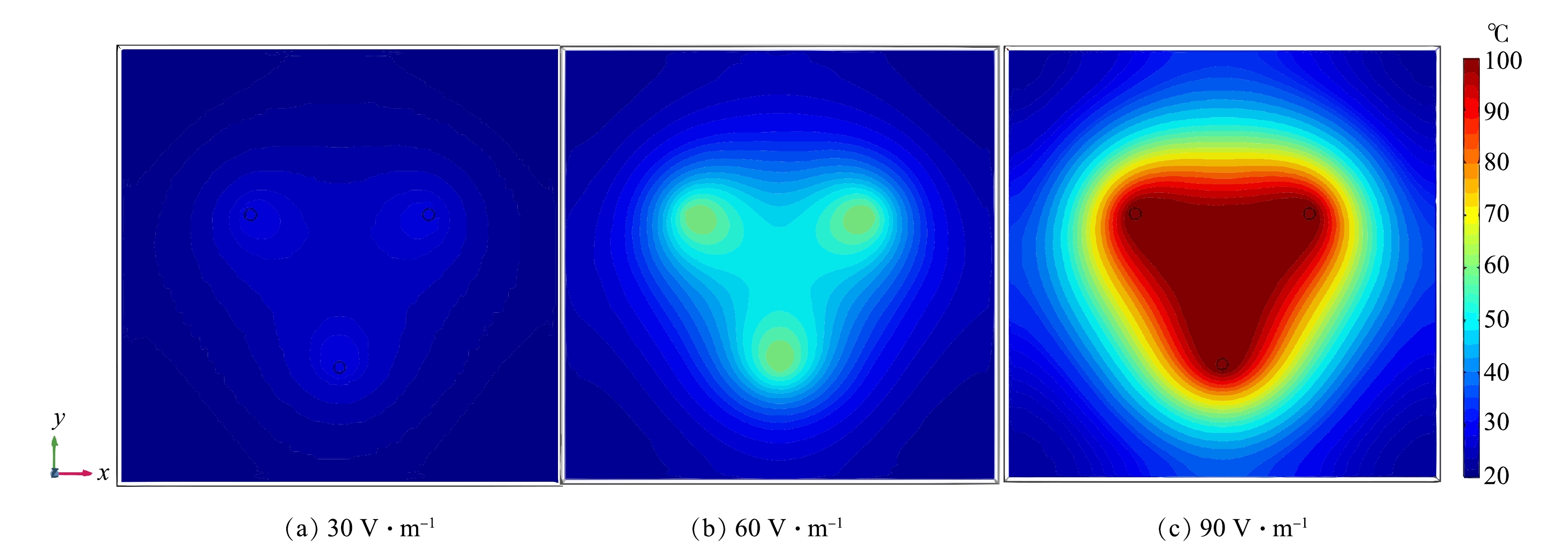
电场强度是影响电阻加热升温速率的重要因素,由电极电压和电极间距共同决定,为了考察场地尺度下的温度场以及各条件对温度场的影响,建立了图1(b)的场地模型。图3和图4分别展示了电极间距6 m时,30、60、90 V·m−1电场强度下加热70 d后,温度场的横截面以及位于3个电极中心点(见图1(b))温度的变化。从图3可以看出,电场强度越大,土壤升温速率越快,电极附近的升温更快,温度更高。这与MCGEE等[10]和HAN等[22]的研究结果是一致的。电流产生的焦耳热随电场强度的增大而增大,电场强度越大,土壤升温速率越快;电极附近的电流密度最高,产生的焦耳热最多,所以土壤升温速率更快。
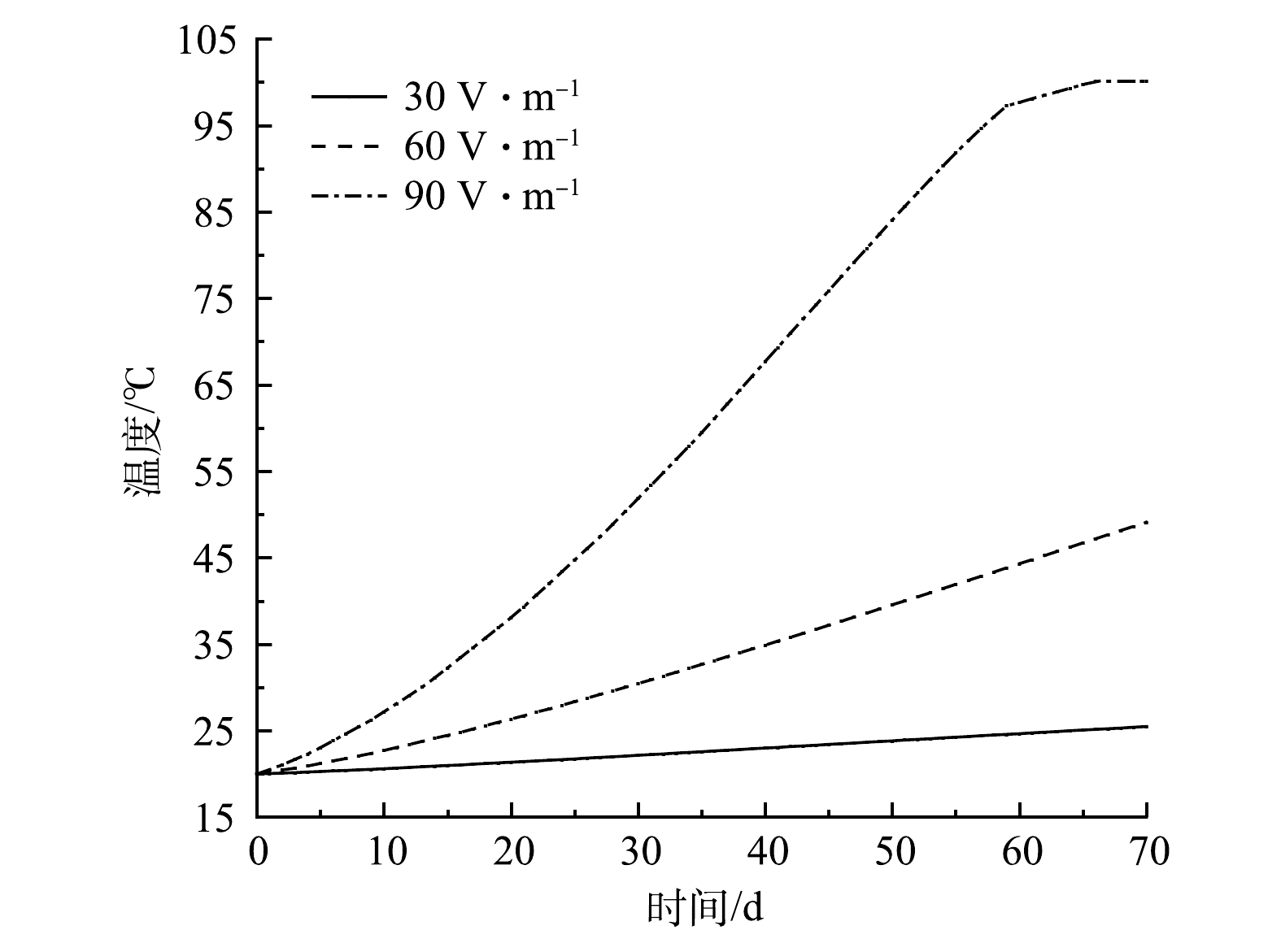
从图4可以看到,场地在加热90 d后,30、60和90 V·m−1电场强度获得的中心温度分别为25.5、49.1和100.0 ℃,中心点的平均升温速率分别为0.079,0.420和1.210 ℃·d−1。由式(1)可知,焦耳热与电场强度的平方呈正比,电场强度为30、60、90 V·m−1时中心点升温速率之比应为1∶4∶9,小于模拟得到的比值,即1∶5.3∶15.5。这说明,中心点的温度可能是电流焦耳热和外部热传导叠加共同决定。此外,电场强度为90 V·m−1时,当中心点温度达到97 ℃后,升温速率明显放缓。这是因为,此时的电极温度已经达到水的沸点,电极土壤水分蒸发带走了大量热量,导致土壤热导率和土壤电导率下降,从而使升温速率下降。
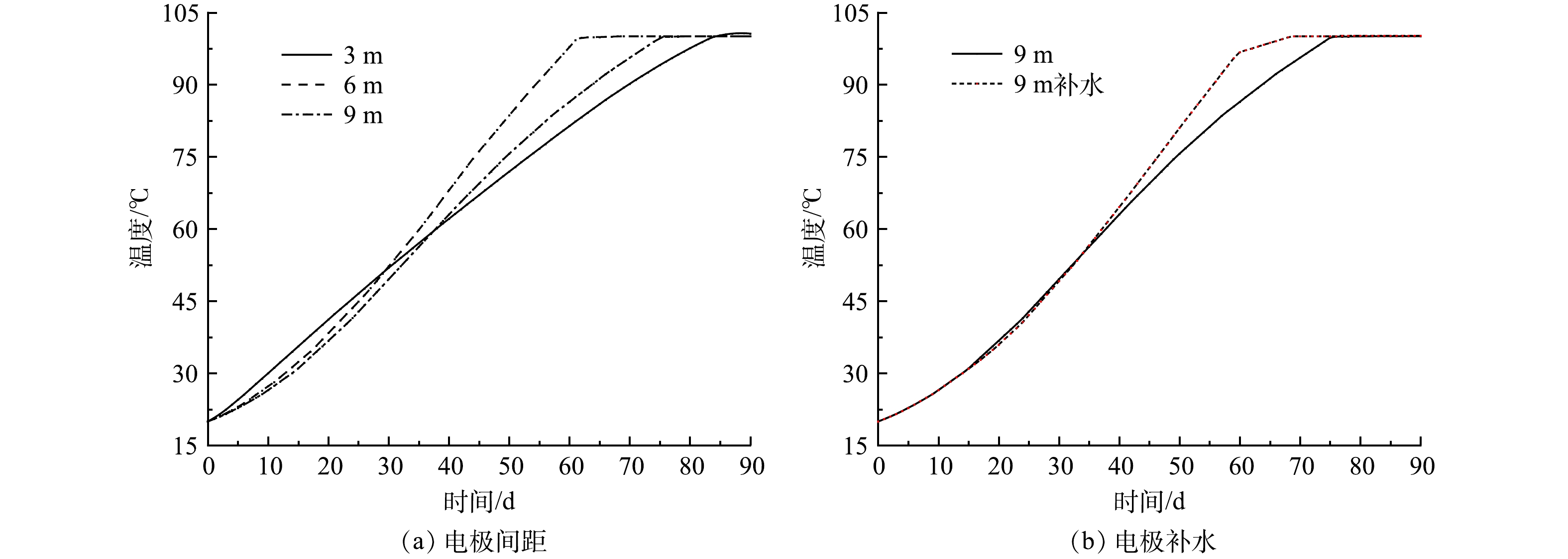
电极间距的设置决定了电极井的数量,会极大地影响修复场地的成本和热传导过程。为探究电极间距这一单因素对电阻加热过程的影响,在保持电场强度90 V·m−1不变的条件下,模拟电极间距3、6和9 m时中心温度的变化,结果如图5所示。从图5(a)可以看出,在电场强度为90 V·m−1,电极间距为3、6和9 m时,中心点的温度达到100 ℃的时间分别为84、62和75 d,达到100 ℃的能耗分别为10 418、23 375和51 311 kW·h。综上可知,6 m为最适宜的电极间距,此时中心点的升温速率最快,且相较于电极间距为3 m时场地修复需要布设的电极井数量更少,相较于电极间距为9 m时需要的能耗更少。从图5(a)还可以看出,随着电极间距的增大,中心点升温速率先增大后减小。这是因为,为了保证电场强度一定,电压随电极间距增大而增大,导致电极处的升温速率也随之增大,这一定程度上弥补了间距增大导致的中心点热传导距离增大的不足。但是,9 m间距下的电压增加,造成电极附近温度过早达到水的沸点,导致电极周围土壤水分过早蒸发,土壤热导率和电导率下降,升温速率略为下降。
为了考察电极附近土壤水分蒸发对中心点升温速率的影响,模拟了电场强度90 V·m−1、电极间距为9 m时,对电极附近补水的情况下,中心点温度的变化。从图5(b)可以看到,在加热40 d后,补水的情况下中心点温度明显高于不补水的情况,补水的情况下中心点的温度达到100 ℃的时间为68 d,比不补水的情况下早8 d。此结果表明,电极周围土壤水分过早蒸发是造成升温速率下降的原因之一,补水可以使土壤含水率增大,增加土壤的电导率和热导率,提高了升温速率。这与葛松等[3]的研究结果一致。监测电极的电流变化,并及时对电极附近进行补水,对于更快加热到目标温度至关重要。
2.3 场地条件下地下水流动对电阻加热的影响
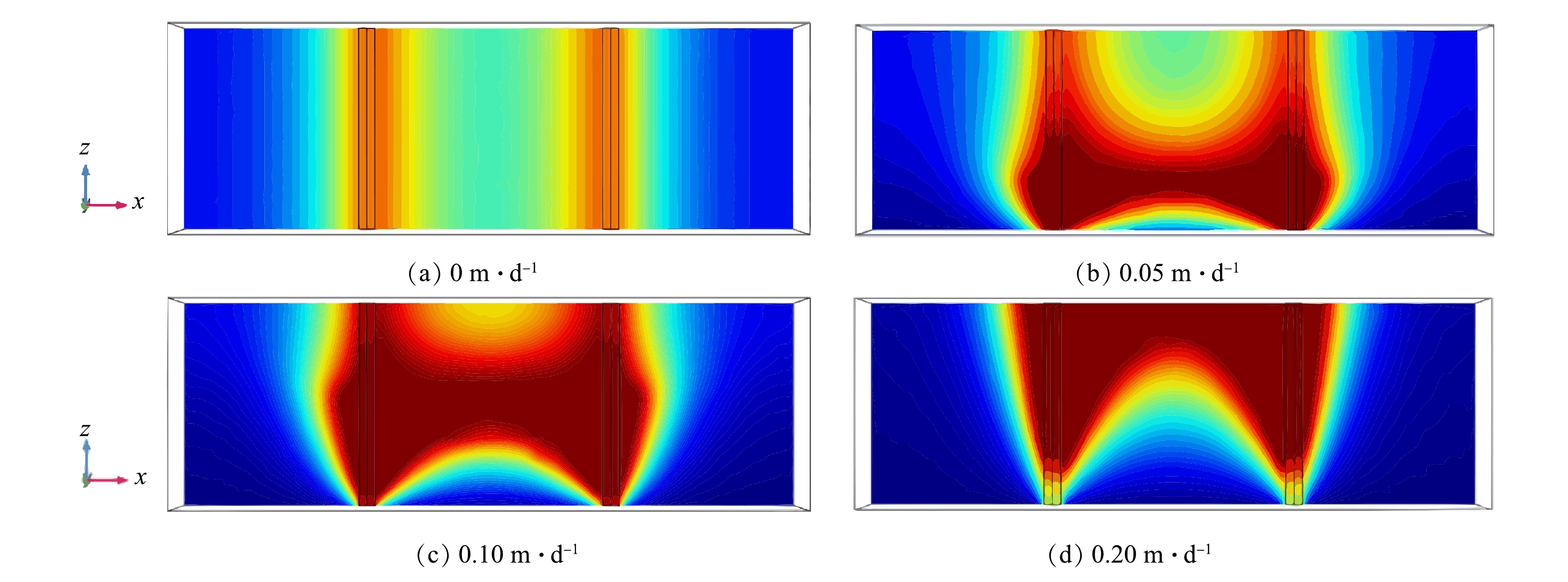
使用原位电阻加热的场地一般修复深度都较大,有必要考虑地下水流动和地下水位变化对温度场的影响。为进行预测,分别设定了0、0.1、0.2、0.3 m·d−1的地下水流速下加热30 d和0、0.05、0.10、0.20 m·d−1的地下水上涨速度下加热30 d的条件进行模拟,流入的地下水出的温度假定为10 ℃,温度场的变化结果如图6和图7所示。从图6可以看到,随着地下水流速的增加,场地左侧的温度逐渐降低到10 ℃,温度场右移的趋势越明显,中心点的最高温度从100.0 ℃降低到38.5 ℃。这说明,较高的地下水流速会将场地中的热量带向下游,对场地升温速率造成不利影响,与MUNHOLLAND等[23]在二维沙箱中得到的结果一致。实际工程中应尽量减小地下水流速,必要时可增加侧向的物理屏障来形成止水帷幕,或通过蒸汽注入等手段来增加流入地下水的温度。
从图7可以看到,随着地下水位上涨速度提升至0.1 m·d−1,场地下部和两侧的温度逐渐降低到10 ℃,但由不饱和区域变为饱和区域的土壤升温速率上升,中心点最高温度从51 ℃升高到100 ℃。随着地下水位上升速度达到0.2 m·d−1,相较于0.1 m·d−1时,温度场明显向上移动,中心点最高温度从100 ℃降低到54 ℃。这是因为,地下水位上涨可以起到一定的补水作用,提高不饱和区域土壤的电导率,从而提高土壤升温速率,但地下水上涨速度过快还是会导致流失的热量多于通过补水增加的焦耳热,使得场地温度从底部开始下降,不利于场地的修复。应当指出,由于实际场地地层的复杂性,地下水的流速和流向并不是均匀的。因此,对于非均质地层,可以建立相应的多模块的空间耦合模型,通过改变各地层模块的物理参数,从而实现数值模拟目标。
3. 结论
1)电阻加热温度场数值模型与土柱装置实验数据吻合度较好,模拟值和实测值均方误差为0.05~12.29,平均相对误差为0.42%~5.32%,数值模型具有较好的预测准确性。
2)场地模型研究发现,升温速率与电场强度成正比,电极处升温速率最快;电场强度为90 V·m−1时6 m为最适宜的电极间距,此时中心点升温速率最快,能耗相对较低,需要的电极井较少;模型考虑水分蒸发的情况下,电极周围土壤水分会更早蒸发,导致土壤升温速率下降,此时对电极附近补水可以显著增加土壤升温速率,故在实际工程中应监测电流和功率,并及时对电极附近进行补水。
3)场地地下水流动会带走热量,不利于场地的热修复。但对于不饱和场地,地下水位上涨速度小于等于0.1 m·d−1时可以起到补水作用,提高场地升温速率。故对于地下水流速过高的场地应采取水力平衡或设置止水帷幕等工程措施以缓解热量流失。
-
[1] Shin J. H., Boo H. O., Bang E., et al. Development of a cleanup method for polybrominated diphenyl ether (PBDE) in fish by freezing-lipid filtration. European Food Research and Technology, 2012, 235(2): 295-301 [2] Haave M., Folven K. I., Carroll T., et al. Cerebral gene expression and neurobehavioural development after perinatal exposure to an environmentally relevant polybrominated diphenylether (BDE47). Cell Biology and Toxicology, 2011, 27(5): 343-361 [3] Darnerud P. O., Wong J., Bergman Å., et al. Common viral infection affects pentabrominated diphenyl ether (PBDE) distribution and metabolic and hormonal activities in mice. Toxicology, 2005, 210(2-3): 159-167 [4] Routti H., Letcher R. J., Chu S. G., et al. Polybrominated diphenyl ethers and their hydroxylated analogues in ringed seals (Phoca hispida) from Svalbard and the Baltic sea. Environmental Science & Technology, 2009, 43(10): 3494-3499 [5] Leung A. O. W., Luksemburg W. J., Wong A. S., et al. Spatial distribution of polybrominated diphenyl ethers and polychlorinated dibenzo-p-dioxins and dibenzofurans in soil and combusted residue at Guiyu, an electronic waste recycling site in southeast China. Environmental Science & Technology, 2007, 41(8): 2730-2737 [6] Darnerud P. O., Risberg S. Tissue localisation of tetra- and pentabromodiphenyl ether congeners (BDE-47, -85 and -99) in perinatal and adult C57BL mice. Chemosphere, 2006, 62(3): 484-493 [7] Branchi I., Capone F., Vitalone A., et al. Early developmental exposure to BDE 99 or aroclor 1254 affects neurobehavioural profile: Interference from the administration route. Neuro Toxicology, 2005, 26(2): 183-192 [8] Li Fei, Xie Qing, Li Xuehau, et al. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-β: In vitro and in silico investigations. Environmental Health Perspectives, 2012, 118(5): 602-606 [9] Wang Xingbao, Wang Yong, Chen Jinwen, et al. Computational toxicological investigation on the mechanism and pathways of xenobiotics metabolized by cytochrome P450: A case of BDE-47. Environmental Science & Technology, 2012, 46(9): 5126-5133 [10] 薛南冬, 李发生. 持久性有机污染物(POPs)污染场地风险控制与环境修复. 北京: 科学出版社, 2011: 5 [11] Booij K., Zegers B. N., Boon J. P. Levels of some polybrominated diphenyl ether (PBDE) flame retardants along the Dutch coast as derived from their accumulation in SPMDs and blue mussels (Mytilus edulis). Chemosphere, 2002, 46(5): 683-688 [12] Wurl O., Lam P. K. S., Obbard J. P. Occurrence and distribution of polybrominated diphenyl ethers (PBDEs) in the dissolved and suspended phases of the sea-surface microlayer and seawater in Hong Kong, China. Chemosphere, 2006, 65(9): 1660-1666 [13] Oros D. R., Hoover D., Rodigari F., et al. Levels and distribution of polybrominated diphenyl ethers in water, surface sediments, and bivalves from the San Francisco Estuary. Environmental Science & Technology, 2005, 39(1): 33-41 [14] De Boer J., Wester P. G., van der Horst A., et al. Polybrominated diphenyl ethers in influents, suspended particulate matter, sediments, sewage treatment plant and effluents and biota from the Netherlands. Environmental Pollution, 2003, 122(1): 63-74 [15] Hale R. C., La Gardia M. J., Harvey E., et al. Brominated flame retardant concentrations and trends in abiotic media. Chemosphere, 2006, 64(2): 181-186 [16] Chen Chang'er, Zhao Hongxia, Chen Jingwen, et al. Polybrominated diphenyl ethers in soils of the modern Yellow River Delta, China: Occurrence, distribution and inventory. Chemosphere, 2012, 88(7): 791-797 [17] 陈社军, 麦碧娴, 曾永平, 等. 珠江三角洲及南海北部海域表层沉积物中多溴联苯醚的分布特征. 环境科学学报, 2005, 25(9): 1265-1271 Chen Shejun, Mai Bixian, Zeng Yongping, et al. Polybrominated diphenyl ethers (PBDEs) in surficial sediments of the Pearl River Delta and adjacent South China Sea. Acta Scientiae Circumstantiae, 2005, 25(9): 1265-1271(in Chinese) [18] Song Wenlu, Ford J. C., Li An, et al. Polybrominated diphenyl ethers in the sediments of the Great Lakes. 1. Lake Superior. Environmental Science & Technology, 2004, 38(12): 3286-3293 [19] Eljarrat E., De La Cal A., Raldua D., et al. Occurrence and bioavailability of polybrominated diphenyl ethers and hexabromocyclododecane in sediment and fish from the Cinca River, a tributary of the Ebro River (Spain). Environmental Science & Technology, 2004, 38(9): 2603-2608 [20] Eljarrat E., Labandeira A., Marsh G. Decabrominated diphenyl ether in river fish and sediment samples collected downstream an industrial park. Chemosphere, 2007, 69(8): 1278-1286 [21] Moon H. B., Kannan K., Choi M., et al. Polybrominated diphenyl ethers (PBDEs) in marine sediments from industrialized bays of Korea. Marine Pollution Bulletin, 2007, 54(9): 1402-1412 [22] Jin Jun, Liu Weizhi, Wang Ying, et al. Levels and distribution of polybrominated diphenyl ethers in plant, shellfish and sediment samples from Laizhou Bay in China. Chemosphere, 2008, 71(6): 1043-1050 [23] Alaee M., Cannon C., Muir D., et al. Spatial distribution and seasonal variation of PBDEs in Arctic and Great Lakes air. Organohalogen Compounds, 2001, 52: 26-29 [24] Wang Yawei, Jiang Guibin, Lam P. K. S., et al. Polybrominated diphenyl ether in the East Asian environment: A critical review. Environment International, 2007, 33(7): 963-973 [25] Law R. J., Allchin C. R., de Boer J., et al. Levels and trends of brominated flame retardants in the European environment. Chemosphere, 2006, 64(2): 187-208 [26] Bohlin P., Jones K. C., Tovalin H., et al. Observations on persistent organic pollutants in indoor and outdoor air using passive polyurethane foam samplers. Atmospheric Environment, 2008, 42(31): 7234-7241 [27] Strandberg B., Dodedr N. G., Basu I., et al. Concentrations and spatial variations of polybrominated diphenyl ethers and other organohalogen compounds in Great Lakes air. Environmental Science & Technology, 2001, 35(6): 1078-1083 [28] Hirai T., Fujimine Y., Watanabe S., et al. Distribution of polybrominated diphenyl ethers in Japanese autopsy tissue and body fluid samples. Environmental Science and Pollution Research, 2012, 19(8): 3538-3546 [29] Johnson-Restrepo B., Kannan K., Addink R., et al. Polybrominated diphenyl ethers and polychlorinated biphenyls in a marine foodweb of coastal Florida. Environmental Science & Technology, 2005, 39(21): 8243-8250 [30] Endo S., Mewburn B., Escher B. I. Liposome and protein-water partitioning of polybrominated diphenyl ethers (PBDEs). Chemosphere, 2013, 90(2): 505-511 [31] Wolkers H., van Bavel B., Derocher A. E., et al. Congener-specific accumulation and food chain transfer of polybrominated diphenyl ethers in two arctic food chains. Environmental Science & Technology, 2004, 38(6): 1667-1674 [32] Ikonomou M. G., Rayne S., Addison R. F. Exponential increases of the brominated flame retardants, polybrominated diphenyl ethers, in the Canadian Arctic from 1981 to 2000. Environmental Science & Technology, 2002, 36(9): 1886-1892 [33] Betts K. S. Flame-proofing the Arctic? Environmental Science & Technology, 2002, 36(9): 188A-192A [34] Akutsu K., Kitagawa M., Nakazawa H., et al. Time-trend (1973-2000) of polybrominated diphenyl ethers in Japanese mother's milk. Chemosphere, 2003, 53(6): 645-654 [35] Corsolini S., Covaci A., Ademollo N., et al. Occurrence of organochlorine pesticides (OCPs) and their enantiomeric signatures, and concentrations of polybrominated diphenyl ethers (PBDEs) in the Adélie penguin food web, Antarctica. Environmental Pollution, 2006, 140(2): 371-382 [36] Wang Yawei, Wang T., Li An, et al. Selection of bioindicators of polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in mollusks in the Chinese Bohai Sea. Environmental Science & Technology, 2008, 42(19): 7159-7165 [37] Li Yingming, Jiang Guibin, Wang Yawei, et al. Concentrations, profiles and gas-particle partitioning of PCDD/Fs, PCBs and PBDEs in the ambient air of an E-waste dismantling area, southeast China. Chinese Science Bulletin, 2008, 53(4): 521-528 [38] Hundt K., Jonas U., Hammer E., et al. Transformation of diphenyl ethers by Trametes versicolor and characterization of ring cleavage products. Biodegradation, 1999, 10(4): 279-286 [39] Rayne S., Ikonomou M. G., Whale M. D. Anaerobic microbial and photochemical degradation of 4, 4'-dibromodiphenyl ether. Water Research, 2003, 37(3): 551-560 [40] Eriksson J., Green N., Marsh G., et al. Photochemical decomposition of 15 polybrominated diphenyl ether congeners in methanol/water. Environmental Science & Technology, 2004, 38(11): 3119-3125 [41] Mas S., De Juan A., Lacorte S., et al. Photodegradation study of decabromodiphenyl ether by UV spectrophotometry and a hybrid hard- and soft-modelling approach. Analytica Chimica Acta, 2008, 618(1): 18-28 [42] Konstantinov A., Bejan D., Bunce N. J., et al. Electrolytic debromination of PBDEs in DE-83TM technical decabromodiphenyl ether. Chemosphere, 2008, 72(8): 1159-1162 [43] Grittini C., Malcomson M., Fernando Q., et al. Rapid dechlorination of polychlorinated biphenyls on the surface of a Pd/Fe bimetallic system. Environmental Science & Technology, 1995,29(11): 2898-2900 [44] Gillham R. W., O'Hannesin S. F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water, 1994, 32(6): 958-967 [45] Johnson T. J., Fish W., Gorby Y. A., et al. Degradation of carbon tetrachloride by iron metal: Complexation effects on the oxide surface. Journal of Contaminant Hydrology, 1998, 29(4): 379-398 [46] Matheson J. L., Tratnyek P. G. Reductive dehalogenation of chlorinated methanes by iron metal. Environmental Science & Technology, 1994, 28(12): 2045-2053 [47] Luckey F., Fowler B., Litten S. Establishing baseline levels of polybrominated diphenyl ethers in Lake Ontario surface waters//Proceedings of the Second International Workshop on Brominated Flame Retardants. Stockholm, Sweden: BFR, 2001 [48] Wong A., Lei Yingduan, Alaee A., et al. Vapor pressures of the polybrominated diphenyl ethers. Journal of Chemical & Engineering Date, 2001, 46(2): 239-242 [49] Dodder N. G., Strandberg B., Hites R. A. Concentrations and spatial variations of polybrominated diphenyl ethers in fish and air from the northeastern United States. Organohalogen Compounds, 2000, 47: 69-72 [50] Arias P. Brominated diphenyloxides as flame retardants. Bromine based chemicals. Draft report of OECD, Brussels, 1992 [51] Nose K., Hashimoto S., Takahashi S., et al. Degradation pathways of decabromodiphenyl ether during hydrothermal treatment. Chemosphere, 2007, 68(1): 120-125 [52] Robrock K. R., Korytár P., Alvarez-Cohen L. Pathways for the anaerobic microbial debromination of polybrominated diphenyl ethers. Environmental Science & Technology, 2008, 42(8): 2845-2852 [53] He Jianzhong, Robrock K. R., Alvarez-Cohen L. Microbial reductive debromination of polybrominated diphenyl ethers (PBDEs). Environmental Science & Technology, 2006, 40(14): 4429-4434 [54] Gerecke A. C., Giger W., Hartmann P. C., et al. Anaerobic degradation of brominated flame retardants in sewage sludge. Chemosphere, 2006, 64(2): 311-317 [55] 张梅, 林匡飞, 刘莉莉, 等. 光源和溶剂对十溴联苯醚光降解的影响. 环境污染与防治, 2009, 31(1): 26-30 Zhang Mei, Lin Kuangfei, Liu Lili, et al. Effects of light sources and solvents on the photodegradation of decabromodiphenyl ether. Environmental Pollution & Control, 2009, 31(1): 26-30(in Chinese) [56] Sánchez-Prado L., González-Barreiro C., Lores M., et al. Photochemical studies of a polybrominated diphenyl ethers (PBDEs) technical mixture by solid phase microextraction (SPME). Chemosphere, 2005, 60(7): 922-928 [57] Olsman H., van Bavel B., Kalbin G., et al. Formation of dioxin-like compounds as photoproducts of decarbominated diphenyl ether (deBDE) during UV-irradiation. Organohalogen Compounds, 2002, 58: 41-44 [58] Hagberg J., Olsman H., van Bavel B., et al. Chemical and toxicological characterisation of PBDFs from photolytic decomposition of decaBDE in toluene. Environment International, 2006, 32(7): 851-857 [59] 赵世岩. 碳纳米管修饰电极对PCBs和PBDEs的电催化还原脱卤. 大连: 大连理工大学硕士学位论文, 2009 Zhao Shiyan. Electrocatalytic reductive dehalogenation of polychlorinated biphenyls and polybrominated diphenyl ethers by modified carbon nanotubes electrode. Dalian: Master Dissertation of Dalian University of Technology, 2009(in Chinese) [60] Bonin P. M. L., Edwards P., Bejan D., et al. Catalytic and electrocatalytic hydrogenolysis of brominated diphenyl ethers. Chemosphere, 2005, 58(7): 961-967 [61] 王世杰, 谷庆宝, 杜平, 等. 零价铁表面积对泥浆反应体系中硝基苯降解行为的影响. 环境科学研究, 2007, 20(6): 106-109 Wang Shijie, Gu Qingbao, Du Pin, et al. Effects of iron surface area on reduction of nitrobenzene-contaminated sediment in slurry reaction system. Environmental Sciences Research, 2007, 20(6): 106-109(in Chinese) [62] Cong Xin, Xue Nandong, Wang Shijie, et al. Reductive dechlorination of organochlorine pesticides in soils from an abandoned manufacturing facility by zero-valent iron. Science of the Total Environment, 2010, 408(16): 3418-3423 [63] Keum Y. S., Li Q. X. Reductive debromination of polybrominated diphenyl ethers by zerovalent iron. Environmental Science & Technology, 2005, 39(7): 2280-2286 [64] Carvalho-Knighton K., Talalaj L., Devor R. PBDE degradation with Zero-valent bimetallic systems//Environmental Applications of Nanoscale and Microscale Reactive Metal Particles. Washington, DC: American Chemical Society, 2009 [65] Fang Zhanqiang, Qiu Xinhong, Chen Jinhong, et al. Debromination of polybrominated diphenyl ethers by Ni/Fe bimetallic nanoparticles: Influencing factors, kinetics, and mechanism. Journal of Hazardous Materials, 2011, 185(2-3): 958-969 [66] Shih Y. H., Tai Y. T. Reaction of decabrominated diphenyl ether by zerovalent iron nanoparticles. Chemosphere, 2010, 78(10): 1200-1206 [67] Feng Jing, Lim T. T. Pathways and kinetics of carbon tetrachloride and chloroform reductions by nano-scale Fe and Fe/Ni particles: Comparison with commercial micro-scale Fe and Zn. Chemosphere, 2005, 59(9): 1267-1277 [68] Xu Xinhua, Zhou Hongyi, He Ping, et al. Catalytic dechlorination kinetics of p-dichlorobenzene over Pd/Fe catalysts. Chemosphere, 2005, 58(8): 1135-1140 [69] Lin C. J., Lo S. L., Liou Y. H. Dechlorination of trichloroethylene in aqueous solution by noble metal-modified iron. Journal of Hazardous Materials, 2004, 116(3): 219-228 [70] Liu Yihui, Yang Fenglin, Chen Jingwen, et al. Linear free energy relationships for dechlorination of aromatic chlorides by Pd/Fe. Chemosphere, 2003, 50(10): 1275-1279 [71] 程荣, 王建龙, 张伟贤. 纳米金属铁降解有机卤化物的研究进展. 化学进展, 2006, 18(1): 93-99 Cheng Rong, Wang Jianlong, Zhang Weixian. The research progress on degradation of halogenated organic compounds by nano iron. Progress in Chemistry, 2006, 18(1): 93-99(in Chinese) [72] Lien H. L., Zhang Weixian. Hydrodechlorination of chlorinated ethanes by nanoscale Pd/Fe bimetallic particles. Journal of Environmental Engineering, 2005, 131(1): 4-10 [73] Lien H. L., Zhang Weixian. Transformation of chlorinated methanes by nanoscale iron particles. Journal of Environmental Engineering, 1999, 125(11): 1042-1047 [74] Choe S., Lee S. H., Chang Y. Y., et al. Rapid reductive destruction of hazardous organic compounds by nanoscale Fe0. Chemosphere, 2001, 42(2): 367-372 [75] Golubina E. V., Lokteva E. S., Lazareva T. S., et al. Hydrodechlorination of tetrachloromethane in the vapor phase in the presence of Pd-Fe/sibunit catalysts. Kinetics and Catalysis, 2004, 45(2): 183-188 [76] Morales J., Hutcheson R., Cheng I. F. Dechlorination of chlorinated phenols by catalyzed and uncatalyzed Fe(0) and Mg(0) particles. Journal of Hazardous Materials, 2002, 90(1): 97-108 [77] Jovanovic G. N., Žnidaršic Plazl P., Sakrittichai P., et al. Dechlorination of p-chlorophenol in a microreactor with bimetallic Pd/Fe catalyst. Industrial & Engineering Chemistry Research, 2005, 44(14): 5099-5106 [78] Liu Yihui, Yang Fenglin, Yue P. F., et al. Catalytic dechlorination of chlorophenols in water by palladium/iron. Water Research, 2001, 35(8): 1887-1890 [79] Wei Jianjun, Xu Xinhua, Liu Yong, et al. Catalytic hydrodechlorination of 2,4-dichlorophenol over nanoscale Pd/Fe: Reaction pathway and some experimental parameters. Water Research, 2006, 40(2): 348-354 [80] Patel U. D., Suresh S. Dechlorination of chlorophenols using magnesium-palladium bimetallic system. Journal of Hazardous Materials, 2007, 147(1-2): 431-438 [81] Zhang Weixian, Wang Chuanbao, Lien H. L. Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catalysis Today, 1998, 40(4): 387-395 [82] Li Fang, Vipulanandan C., Mohanty K. K. Microemulsion and solution approaches to nanoparticle iron production for degradation of trichloroethylene. Colloids and Surfaces A, 2003, 223(1-3): 103-112 [83] Schrick B., Blough J. L., Jones A. D., et al. Hydrodechlorination of trichloroethylene to hydrocarbons using bimetallic nickel-iron nanoparticles. Chemistry of Materials, 2002, 14(12): 5140-5147 [84] Wang Chuanbao, Zhang Weixian. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environmental Science & Technology, 1997, 31(7): 2154-2156 [85] Agarwal S., Al-Abed S. R., Dionysiou D. D. Enhanced corrosion-based Pd/Mg bimetallic systems for dechlorination of PCBs. Environmental Science & Technology, 2007, 41(10): 3722-3727 [86] Choi H., Al-Abed S. R., Agarwal S., et al. Synthesis of reactive nano-Fe/Pd bimetallic system-impregnated activated carbon for the simultaneous adsorption and dechlorination of PCBs. Chemistry of Materials, 2008, 20(11): 3649-3655 [87] Yak H. K., Lang Qingyong, Wai C. M. Relative resistance of positional isomers of polychlorinated biphenyls toward reductive dechlorination by zerovalent iron in subcritical water. Environmental Science & Technology, 2000, 34(13): 2792-2798 [88] Qiu Xinhong, Fang Zhanqiang, Liang Bin, et al. Degradation of decabromodiphenyl ether by nano zero-valent iron immobilized in mesoporous silica microspheres. Journal of Hazardous Materials, 2011, 193: 70-81 [89] Peng Y. H., Chen K. C., Shih Y. H. Adsorption and sequential degradation of polybrominated diphenyl ethers with zerovalent iron. Journal of Hazardous Materials, 2013, 260: 844-850 [90] Hu Jiwei, Zhuang Yuan, Luo Jin, et al. A theoretical study on reductive debromination of polybrominated diphenyl ethers. International Journal of Molecular Sciences, 2012, 13(7): 9332-9342 [91] Yu Kai, Gu Cheng, Boyd S. A., et al. Rapid and extensive debromination of decabromodiphenyl ether by smectite clay-templated subnanoscale zero-valent iron. Environmental Science & Technology, 2012, 46(16): 8969-8975 [92] Zhuang Yuan, Ahn S., Luthy R. G. Debromination of polybrominated diphenyl ethers by nanoscale zerovalent iron: pathways, kinetics, and reactivity. Environmental Science & Technology, 2010, 44(21): 8236-8242 [93] Pfeifer F., Schacht S., Klein J., et al. Degradation of diphenylether by Pseudomonas cepacia. Archives of Microbiology, 1989, 152(6): 515-519 [94] Schmidt S., Wittich R. M., Erdmann D., et al. Biodegradation of diphenyl ether and its monohalogenated derivatives by Sphingomonas sp. strain SS3. Applied and Environmental Microbiology, 1992, 58(9): 2744-2750 [95] Schmidt S., Fortnagel P., Wittich R. M. Biodegradation and transformation of 4,4'-and 2,4-dihalodiphenyl ethers by Sphingomonas sp. strain SS3. Applied and Environmental Microbiology, 1993, 59(11): 3931-3933 [96] Kajiwara N., Noma Y., Takigami H. Photolytic debromination of deca-BDE and DBDPE in flame-retarded plastics. Organohalogen Compounds, 2007, 69: 924-927 [97] Watanabe I., Tatsukawa R. Formation of brominated dibenzofurans from the photolysis of flame retardant decabromobiphenyl ether in hexane solution by UV and sun light. Bulletin of Environmental Contamination and Toxicology, 1987, 39(6): 953-959 [98] 李长芳, 胡勇有, 黄国富. 纳米Pd/Fe催化甲醇/水中2, 2', 4, 4'-四溴联苯醚(BDE-47)还原脱溴. 环境科学学报, 2012, 32(10): 2353-2359 Li Changfang, Hu Yongyou, Huang Guofu. Catalytic debromination of 2,2', 4,4'-Tetrabromodiphenylether ( BDE-47) by Pd/Fe nanoparticles in methanol/water. Acta Scientiae Circumstantiae, 2012, 32(10): 2353-2359(in Chinese) [99] 全燮, 刘会娟, 杨凤林, 等. 二元金属体系对水中多氯有机物的催化还原脱氯特性. 中国环境科学, 1998, 18(4): 333-336 Quan Xie, Liu Huijuan, Yang Fenglin, et al. Dechlorination of three polychlorinated hydrocarbons in water using bimetallic system. China Environmental Science, 1998, 18(4): 333-336(in Chinese) [100] Cheng I. F., Fernando Q., Korte N. Electrochemical dechlorination of 4-chlorophenol to phenol. Environmental Science & Technology, 1997, 31(4): 1074-1078 [101] 曹声春, 胡艾希, 尹笃林. 催化原理及其工业应用技术. 长沙: 湖南大学出版社, 2001 [102] Korte N. E., Zutman J. L., Schlosser R. M., et al. Field application of palladized iron for the dechlorination of trichloroethene. Waste Management, 2000, 20(8): 687-694 [103] Cwiertny D. M., Bransfield S. J., Livi K. J., et al. Exploring the influence of granular iron additives on 1,1,1-trichloroethane reduction. Environmental Science & Technology, 2006, 40(21): 6837-6843 [104] Luo Si, Yang Shaogui, Sun Cheng, et al. Improved debromination of polybrominated diphenyl ethers by bimetallic iron-silver nanoparticles coupled with microwave energy. Science of the Total Environment, 2012, 429: 300-308 [105] Li An, Tai Chao, Zhao Zongshan, et al. Debromination of decabrominated diphenyl ether by resin-bound iron nanoparticles. Environmental Science & Technology, 2007, 41(19): 6841-6846 [106] 梁贺升, 陈少瑾. 纳米零价铁脱溴十溴联苯醚(BDE-209)的研究. 韩山师范学院学报, 2010, 31(3): 70-73 Liang Hesheng, Chen Shaojin. Debromination decabromodiphenyl (BDE-209)by nano zero-valent iron. Journal of Hanshan Normal University, 2010, 31(3): 70-73(in Chinese) [107] 明磊强, 何义亮, 章敏, 等. 零价铁降解多溴联苯醚影响条件的研究. 净水技术, 2010, 29(2): 49-52 Ming Leiqiang, He Yiliang, Zhang Min, et al. Studies on effect conditions for removal of polybrominated diphenyl ethers by zero valent iron. Water Purification Technology, 2010, 29(2): 49-52(in Chinese) -

 点击查看大图
点击查看大图
计量
- 文章访问数: 3310
- HTML全文浏览数: 2753
- PDF下载数: 1611
- 施引文献: 0



 下载:
下载: